K-252cProtein kinase inhibitor CAS# 85753-43-1 |
- CX-4945 (Silmitasertib)
Catalog No.:BCC3693
CAS No.:1009820-21-6
- GF 109203X
Catalog No.:BCC3704
CAS No.:133052-90-1
- Go 6983
Catalog No.:BCC3705
CAS No.:133053-19-7
- Go 6976
Catalog No.:BCC3703
CAS No.:136194-77-9
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 85753-43-1 | SDF | Download SDF |
| PubChem ID | 3815 | Appearance | Powder |
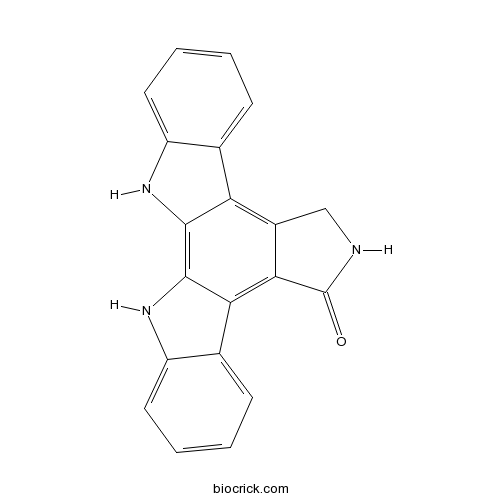
| Formula | C20H13N3O | M.Wt | 311.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Staurosporinone | ||
| Solubility | Soluble to 25 mM in DMSO | ||
| SMILES | C1C2=C3C4=CC=CC=C4NC3=C5C(=C2C(=O)N1)C6=CC=CC=C6N5 | ||
| Standard InChIKey | MEXUTNIFSHFQRG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H13N3O/c24-20-17-12(9-21-20)15-10-5-1-3-7-13(10)22-18(15)19-16(17)11-6-2-4-8-14(11)23-19/h1-8,22-23H,9H2,(H,21,24) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of protein kinase C (IC50 = 2.45 μM) that displays ~ 10-fold selectivity over protein kinase A (IC50 = 25.7 μM). Also inhibits β-lactamase, malate dehydrogenase and chymotrypsin (IC50 values are 8, 8 and 10 μM respectively). Exhibits antiviral activity against GCV-sensitive and -resistant strains of human cytomegalovirus (HCMV) (IC50 values range from 0.13 - 0.32 μM). Shows no activity against herpes simplex virus (HSV). |

K-252c Dilution Calculator

K-252c Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2119 mL | 16.0596 mL | 32.1192 mL | 64.2385 mL | 80.2981 mL |
| 5 mM | 0.6424 mL | 3.2119 mL | 6.4238 mL | 12.8477 mL | 16.0596 mL |
| 10 mM | 0.3212 mL | 1.606 mL | 3.2119 mL | 6.4238 mL | 8.0298 mL |
| 50 mM | 0.0642 mL | 0.3212 mL | 0.6424 mL | 1.2848 mL | 1.606 mL |
| 100 mM | 0.0321 mL | 0.1606 mL | 0.3212 mL | 0.6424 mL | 0.803 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
K-252c is a cell-permeable, reversible, and ATP-competitive inhibitor of protein kinase C (PKC) and protein kinase A (PKA) with IC50 value of 2.45 μM and 25.7 μM, respectively. This component was inhibits β-lactamase, malate dehydrogenase and chymotrypsin with IC50 values of 8, 8 and 10 μM respectively [1].
Protein kinase is known as kinase enzymes that modify other proteins by chemically adding phosphate groups to them (phosphorylation). Phosphorylation can result in a functional change of the target protein (substrate) by changing enzyme activity, cellular location, or association with other proteins in the cells. PKA and PKC are important family member of AGC kinases.
K-252a was previously shown to block nerve growth factor (NGF)-induced neurite outgrowth and the changes in protein phosphorylation elicited by NGF in PC12h cells [2].
The effect of K-252c was also investigated on the severe combined immunodeficient (SCID) mouse-human skin model of psoriasis. It was shown that psoriasis significantly improved after 2 weeks treatment of K-252c [3].
References:
1. Fabre S, Prudhomme M, Rapp M. Protein kinase C inhibitors; structure-activity relationships in K252c-related compounds. Bioorg Med Chem 1993,1:193-196.
2. Hashimoto S. K-252a, a potent protein kinase inhibitor, blocks nerve growth factor-induced neurite outgrowth and changes in the phosphorylation of proteins in PC12h cells. J Cell Biol 1988,107:1531-1539.
3. Raychaudhuri SP, Sanyal M, Weltman H, Kundu-Raychaudhuri S. K252a, a high-affinity nerve growth factor receptor blocker, improves psoriasis: an in vivo study using the severe combined immunodeficient mouse-human skin model. J Invest Dermatol 2004,122:812-819.
- IPI-504 (Retaspimycin hydrochloride)
Catalog No.:BCC2126
CAS No.:857402-63-2
- Retaspimycin
Catalog No.:BCC1889
CAS No.:857402-23-4
- Isoflavidinin
Catalog No.:BCN7604
CAS No.:85734-02-7
- 3-Hydroxysarpagine
Catalog No.:BCN4566
CAS No.:857297-90-6
- PF 915275
Catalog No.:BCC7631
CAS No.:857290-04-1
- TMC353121
Catalog No.:BCC2004
CAS No.:857066-90-1
- WP1066
Catalog No.:BCC2194
CAS No.:857064-38-1
- Scopine HCl
Catalog No.:BCC4940
CAS No.:85700-55-6
- (3S,3'R,8R,9R,9As)-8-methoxy-3'-methyl-3-[(2S,4S)-4-methyl-5-oxooxolan-2-yl]spiro[1,2,3,5,6,7,8,9a-octahydropyrrolo[1,2-a]azepine-9,5'-oxolane]-2'-one
Catalog No.:BCC9250
CAS No.:85700-47-6
- alpha-Conidendrin
Catalog No.:BCN4407
CAS No.:85699-62-3
- (-)-Blebbistatin
Catalog No.:BCC4375
CAS No.:856925-71-8
- Tedizolid
Catalog No.:BCC1990
CAS No.:856866-72-3
- AT7867
Catalog No.:BCC2536
CAS No.:857531-00-1
- PSN632408
Catalog No.:BCC5408
CAS No.:857652-30-3
- Longistylumphylline A
Catalog No.:BCN4408
CAS No.:857672-34-5
- Alstolenine
Catalog No.:BCN4808
CAS No.:85769-33-1
- Polygalaxanthone XI
Catalog No.:BCN7366
CAS No.:857859-82-6
- Motesanib Diphosphate (AMG-706)
Catalog No.:BCC2477
CAS No.:857876-30-3
- 11,12-Di-O-acetyltenacigenin B
Catalog No.:BCN4565
CAS No.:857897-01-9
- (-)-Quinpirole hydrochloride
Catalog No.:BCC6917
CAS No.:85798-08-9
- 3-O-Benzyl estrone
Catalog No.:BCC8638
CAS No.:858-98-0
- Vialinin A
Catalog No.:BCC2367
CAS No.:858134-23-3
- Marsdenoside F
Catalog No.:BCN4564
CAS No.:858360-61-9
- 1,3-Dipropyl-8-phenylxanthine
Catalog No.:BCC6664
CAS No.:85872-53-3
Synthesis of Pyrrolidin-2-ones and of Staurosporine Aglycon (K-252c) by Intermolecular Michael Reaction.[Pubmed:11674542]
J Org Chem. 1999 Jun 25;64(13):4697-4704.
Indolo[2,3-a]pyrrolo[3,4-c]carbazoles were isolated from nature, e.g., from low plants, especially fungi, as structurally rare natural substances. Responsible for naming and also the most important representative of this type is staurosporine (1), isolated from Streptomyces staurosporeus, and its aglycon (2), also known as staurosporinone or K-252c. 3,4-Disubstituted pyrrolidin-2-ones, a group of compounds with many interesting biological properties are related to staurosporinone. The most important property is the inhibition of protein kinase C (PKC), so that this antiproliferative agent can interfere with the cell cycle. The synthetic strategy, developed by us, allows the synthesis of pyrrolidin-2-ones by an intermolecular Michael addition, starting from nitroethene derivatives and substituted acetate Michael donors. With this method also enantioselective syntheses can be carried out using chiral auxiliaries. After reduction of the nitro group and subsequent lactamization, the lactam partial structure, which is essential for the biological activity, is obtained. Besides indole substituents, which were used for the synthesis of staurosporinone, substituted indole-, phenyl-, and pyridyl- as well as enantiomerically pure (S)-proline derivatives were used. Here, considerably high diastereoselectivity and enantioselectivity ((S)-pyrrolidine) could be detected. Just like the total synthesis of staurosporinone within three steps, the easiest and shortest approach reported up to now, with good to moderate yields, this sequence allows highly diastereoselective syntheses, which open the easy access to a new family of compounds.
Kinase inhibitors: not just for kinases anymore.[Pubmed:12672248]
J Med Chem. 2003 Apr 10;46(8):1478-83.
Kinase inhibitors are widely employed as biological reagents and as leads for drug design. Their use is often complicated by their lack of specificity. Although binding conserved ATP sites accounts for some of their nonspecificity, some compounds inhibit proteins not known to bind ATP. It has been found that promiscuous hits from high-throughput screening may act as aggregates. To explore whether this mechanism might explain the action of widely used nonspecific kinase inhibitors, 15 such compounds were studied. Eight of these, rottlerin, quercetin, K-252c, bisindolylmaleimide I, bisindolylmaleimide IX, U0126, indirubin, and indigo, inhibited three diverse non-kinase enzymes. Inhibition was time-dependent and sensitive to enzyme concentration; by light scattering, the compounds formed particles of 100-1000 nm diameter. These observations suggest that these eight kinase inhibitors, at least at micromolar concentrations, are promiscuous and act as aggregates. Results obtained from the use of these compounds at micromolar or higher concentrations against individual enzymes should be interpreted cautiously.
Indolocarbazoles exhibit strong antiviral activity against human cytomegalovirus and are potent inhibitors of the pUL97 protein kinase.[Pubmed:11080540]
Antiviral Res. 2000 Oct;48(1):49-60.
We have analyzed a panel of protein kinase inhibitors (PKIs) and found that some indolocarbazoles (Go6976, K252a, K252c) proved to be highly effective inhibitors of GCV-sensitive and -resistant human cytomegalovirus (HCMV) strains, but did not show any effect against herpes simplex virus. Antiviral activity was determined by focus reduction assays (IC(50) ranging from 0.009 to 0.4 microM). Other inhibitors of serine/threonine kinases (Go6850, H-7, roscovitine) were found to be ineffective. Virus yield at 5 days after infection was reduced by three orders of magnitude with nanomolar concentrations of the indolocarbazoles. These compounds were fully effective when added up to 24 h post infection and showed reduced activity up to 72 h post infection. Cytotoxicity assays in proliferating and non-proliferating cells demonstrated that the effective antiviral concentration of these compounds was significantly lower than either antiproliferative (IC(50)/CC(50) ranging from 6.5 to 390) or cytotoxic (IC(50)/CC(50) ranging from 72. 5 to 1000) doses. The effects of PKIs on the virus-encoded protein kinase pUL97 were studied using recombinant vaccinia viruses. Indolocarbazoles strongly inhibited both pUL97 autophosphorylation (IC(50) ranging from 0.0012 to 0.013 microM) and pUL97-dependent ganciclovir phosphorylation (IC(50) ranging from 0.05 to 0.26 microM). Other inhibitors of serine/threonine kinases showed only weak (Go6850) or no (H-7, roscovitine) effect on these pUL97 functions, while oxoflavone tyrosine kinase inhibitors had no effect at all.
Protein kinase C inhibitors; structure-activity relationships in K252c-related compounds.[Pubmed:8081852]
Bioorg Med Chem. 1993 Sep;1(3):193-6.
K252c-related compounds were synthesized with different framework flexibilities and different functions (imide, amide and amide-alcohol) on the non-indolic heterocycle. The inhibitory activities towards protein kinase C and protein kinase A are compared.


