CX-4945 (Silmitasertib)CK2 inhibitor CAS# 1009820-21-6 |
- XL413
Catalog No.:BCC4241
CAS No.:1169558-38-6
- XL413 hydrochloride
Catalog No.:BCC4039
CAS No.:1169562-71-3
- TBB
Catalog No.:BCC1988
CAS No.:17374-26-4
- TTP 22
Catalog No.:BCC2017
CAS No.:329907-28-0
- Ellagic acid
Catalog No.:BCN5533
CAS No.:476-66-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1009820-21-6 | SDF | Download SDF |
| PubChem ID | 24748573 | Appearance | Powder |
| Formula | C19H12ClN3O2 | M.Wt | 349.77 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Silmitasertib | ||
| Solubility | DMSO : ≥ 35 mg/mL (100.07 mM) 0.1 M NaOH : 33.33 mg/mL (95.29 mM; ultrasonic and adjust pH to 9 with NaOH) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
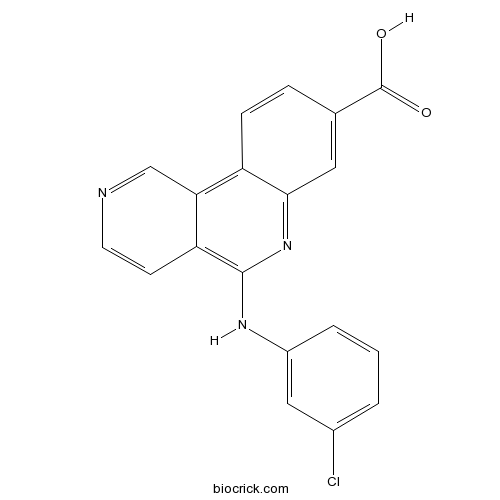
| Chemical Name | 5-(3-chloroanilino)benzo[c][2,6]naphthyridine-8-carboxylic acid | ||
| SMILES | C1=CC(=CC(=C1)Cl)NC2=C3C=CN=CC3=C4C=CC(=CC4=N2)C(=O)O | ||
| Standard InChIKey | MUOKSQABCJCOPU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CX-4945 (Silmitasertib) is a potent and selective inhibitor of CK2 (casein kinase 2) with IC50 of 1 nM. | |||||
| Targets | CK2α | CK2α' | ||||
| IC50 | 1 nM | 1 nM | ||||
| Cell experiment [1]: | |
| Cell lines | Jurkat cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 4d; IC50=0.1 μM |
| Applications | CK2 inhibition was confirmed by measuring the phosphorylation level of the CK2 specific phosphorylation site on Akt (S129). CX-4945 induced dephosphorylation of Akt (S129) and a rapid dephosphorylation of the Akt substrate p21 (T145). Apoptosis was induced by CX-4945. CX-4945 was also found to potently inhibit endogenous intracellular CK2 activity with an IC50 of 0.1 μM in Jurkat cells. |
| Animal experiment [1]: | |
| Animal models | Athymic mice |
| Dosage form | 75 mg/kg; bid; oral taken |
| Application | CX-4945 was tested for in vivo efficacy in established human prostate PC3 xenograft model in athymic mice. Mice bearing subcutaneous PC3 tumors were treated with CX-4945 (25 mg/kg, 50 mg/kg, and 75 mg/kg, p.o, bid). CX-4945 demonstrated tumor growth inhibition (TGI = 19%, 40%, and 86%, respectively) compared to vehicle treated control, and a dose responsive efficacy was observed. Last, CX-4945 was well tolerated in mice as assessed by minimal changes in body weight during the course of treatment compared to vehicle control. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Pierre F, Chua P C, O’Brien S E, et al. Discovery and SAR of 5-(3-chlorophenylamino) benzo [c][2, 6] naphthyridine-8-carboxylic acid (CX-4945), the first clinical stage inhibitor of protein kinase CK2 for the treatment of cancer[J]. Journal of medicinal chemistry, 2010, 54(2): 635-654. | |

CX-4945 (Silmitasertib) Dilution Calculator

CX-4945 (Silmitasertib) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.859 mL | 14.2951 mL | 28.5902 mL | 57.1804 mL | 71.4755 mL |
| 5 mM | 0.5718 mL | 2.859 mL | 5.718 mL | 11.4361 mL | 14.2951 mL |
| 10 mM | 0.2859 mL | 1.4295 mL | 2.859 mL | 5.718 mL | 7.1476 mL |
| 50 mM | 0.0572 mL | 0.2859 mL | 0.5718 mL | 1.1436 mL | 1.4295 mL |
| 100 mM | 0.0286 mL | 0.143 mL | 0.2859 mL | 0.5718 mL | 0.7148 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CX-4945 (Silmitasertib) is a potent and selective casein kinase 2 (CK2) inhibitor with IC50 value of 1 nM. It is ATP-competitive and can be taken orally [1].
CX-4945 has been reported to have antiproliferative activity against a wide range of tumor cell lines. It is suggested that CX-4945 suppresses the CK2 regulated PI3K/Akt signaling pathway by inhibiting Akt phosphorylation at Serine 129, but not by activating PTEN. Additionally, cells treated with CX-4945 had a reduction of p21 phophorylation and an up-regulations of total p21 and p27. CX-4945 has been shown to induce cell-cycle arrest at G2/M phase in breast cancer cell line BT-474. It also causes cell-cycle arrest at G1 phase the breast cancer cell line BxPC-3) [1].
In CX-4945 and BxPC-3 derived mouse xenograft model, CX-4945 induced a reduction of phos-p21 expression along with anti-carcinoma effects [1]
References:
[1] Siddiqui-Jain A1, Drygin D, Streiner N, Chua P, Pierre F, O'Brien SE, Bliesath J, Omori M, Huser N, Ho C, Proffitt C, Schwaebe MK, Ryckman DM, Rice WG,Anderes K. CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy. Cancer Res. 2010 Dec 15;70(24):10288-98.
- NSC 687852 (b-AP15)
Catalog No.:BCC2389
CAS No.:1009817-63-3
- Thiamet G
Catalog No.:BCC4864
CAS No.:1009816-48-1
- Ebrotidine
Catalog No.:BCC1542
CAS No.:100981-43-9
- Rotundine
Catalog No.:BCN5983
CAS No.:10097-84-4
- 2,2-Bis(hydroxymethyl)butyric acid
Catalog No.:BCC8495
CAS No.:10097-02-6
- Stachyose
Catalog No.:BCN2566
CAS No.:10094-58-3
- Caulophyllumine A
Catalog No.:BCN7928
CAS No.:1009318-60-8
- AZD2014
Catalog No.:BCC3732
CAS No.:1009298-59-2
- AZD8055
Catalog No.:BCC3629
CAS No.:1009298-09-2
- Panamycin 607
Catalog No.:BCN1813
CAS No.:100905-89-3
- Piceatannol
Catalog No.:BCN5824
CAS No.:10083-24-6
- (RS)-CPP
Catalog No.:BCC6561
CAS No.:100828-16-8
- Levofloxacin
Catalog No.:BCC4791
CAS No.:100986-85-4
- CY 208-243
Catalog No.:BCC6991
CAS No.:100999-26-6
- Pyridostigmine Bromide
Catalog No.:BCC4579
CAS No.:101-26-8
- Hyoscyamine
Catalog No.:BCN1946
CAS No.:101-31-5
- Bis[4-(dimethylamino)phenyl]methane
Catalog No.:BCC8889
CAS No.:101-61-1
- MK-5108 (VX-689)
Catalog No.:BCC2176
CAS No.:1010085-13-8
- Microcystin-LR
Catalog No.:BCC5339
CAS No.:101043-37-2
- Larixinol
Catalog No.:BCN6484
CAS No.:101046-79-1
- Tenovin-3
Catalog No.:BCC3889
CAS No.:1011301-27-1
- 3,8'-Biapigenin
Catalog No.:BCN5825
CAS No.:101140-06-1
- Milnacipran HCl
Catalog No.:BCC4922
CAS No.:101152-94-7
- Odoriflavene
Catalog No.:BCN8240
CAS No.:101153-41-7
CK2 blockade causes MPNST cell apoptosis and promotes degradation of beta-catenin.[Pubmed:27448963]
Oncotarget. 2016 Aug 16;7(33):53191-53203.
Malignant peripheral nerve sheath tumors (MPNSTs) are soft tissue sarcomas that are a major cause of mortality of Neurofibromatosis type 1 (NF1) patients. MPNST patients have few therapeutic options available and only complete surgical resection can be curative. MPNST formation and survival are dependent on activated beta-catenin signaling. The goal of this study was to determine if inhibition of the CK2 enzyme can be therapeutically exploited in MPNSTs, given CK2's role in mainta ining oncogenic phenotypes including stabilization of beta-catenin. We found that CK2alpha is over-expressed in MPNSTs and is critical for maintaining cell survival, as the CK2 inhibitor, CX-4945 (Silmitasertib), and shRNA targeting CK2alpha each significantly reduce MPNST cell viability. These effects were preceded by loss of critical signaling pathways in MPNSTs, including destabilization of beta-catenin and TCF8. CX-4945 administration in vivo slowed tumor growth and extends survival time. We conclude that CK2 inhibition is a promising approach to blocking beta-catenin in MPNST cells, although combinatorial therapies may be required for maximal efficacy.
Protein kinase CK2 is widely expressed in follicular, Burkitt and diffuse large B-cell lymphomas and propels malignant B-cell growth.[Pubmed:25788269]
Oncotarget. 2015 Mar 30;6(9):6544-52.
Serine-threonine kinase CK2 is highly expressed and pivotal for survival and proliferation in multiple myeloma, chronic lymphocytic leukemia and mantle cell lymphoma. Here, we investigated the expression of alpha catalytic and beta regulatory CK2 subunits by immunohistochemistry in 57 follicular (FL), 18 Burkitt (BL), 52 diffuse large B-cell (DLBCL) non-Hodgkin lymphomas (NHL) and in normal reactive follicles. In silico evaluation of available Gene Expression Profile (GEP) data sets from patients and Western blot (WB) analysis in NHL cell-lines were also performed. Moreover, the novel, clinical-grade, ATP-competitive CK2-inhibitor CX-4945 (Silmitasertib) was assayed on lymphoma cells. CK2 was detected in 98.4% of cases with a trend towards a stronger CK2alpha immunostain in BL compared to FL and DLBCL. No significant differences were observed between Germinal Center B (GCB) and non-GCB DLBCL types. GEP data and WB confirmed elevated CK2 mRNA and protein levels as well as active phosphorylation of specific targets in NHL cells. CX-4945 caused a dose-dependent growth-arresting effect on GCB, non-GCB DLBCL and BL cell-lines and it efficiently shut off phosphorylation of NF-kappaB RelA and CDC37 on CK2 target sites. Thus, CK2 is highly expressed and could represent a suitable therapeutic target in BL, FL and DLBCL NHL.
Adult B-cell acute lymphoblastic leukemia cells display decreased PTEN activity and constitutive hyperactivation of PI3K/Akt pathway despite high PTEN protein levels.[Pubmed:24561792]
Haematologica. 2014 Jun;99(6):1062-8.
Adult B-cell acute lymphoblastic leukemia remains a major therapeutic challenge, requiring a better characterization of the molecular determinants underlying disease progression and resistance to treatment. Here, using a phospho-flow cytometry approach we show that adult diagnostic B-cell acute lymphoblastic leukemia specimens display PI3K/Akt pathway hyperactivation, irrespective of their BCR-ABL status and despite paradoxically high basal expression of PTEN, the major negative regulator of the pathway. Protein kinase CK2 is known to phosphorylate PTEN thereby driving PTEN protein stabilization and concomitant PTEN functional inactivation. In agreement, we found that adult B-cell acute lymphoblastic leukemia samples show significantly higher CK2 kinase activity and lower PTEN lipid phosphatase activity than healthy controls. Moreover, the clinical-grade CK2 inhibitor CX-4945 (Silmitasertib) reversed PTEN levels in leukemia cells to those observed in healthy controls, and promoted leukemia cell death without significantly affecting normal bone marrow cells. Our studies indicate that CK2-mediated PTEN posttranslational inactivation, associated with PI3K/Akt pathway hyperactivation, are a common event in adult B-cell acute lymphoblastic leukemia and suggest that CK2 inhibition may constitute a valid, novel therapeutic tool in this malignancy.
The casein kinase 2 inhibitor, CX-4945, as an anti-cancer drug in treatment of human hematological malignancies.[Pubmed:25873900]
Front Pharmacol. 2015 Mar 31;6:70.
The casein kinase 2 (CK2) protein kinase is a pro-survival kinase and therapeutic target in treatment of various human cancers. CK2 overexpression has been demonstrated in hematological malignancies, including chronic lymphocytic leukemia, chronic myeloid leukemia, acute lymphoblastic leukemia, acute myeloid leukemia, and multiple myeloma. CX-4945, also known as Silmitasertib, is an orally administered, highly specific, ATP-competitive inhibitor of CK2. CX-4945 induces cytotoxicity and apoptosis and is currently being evaluated in clinical trials for treatment of many cancer types. In the past 2 years, the focus on the therapeutic potential of CX-4945 has shifted from solid tumors to hematological malignancies. CX-4945 exerts anti-proliferative effects in hematological tumors by downregulating CK2 expression and suppressing activation of CK2-mediated PI3K/Akt/mTOR signaling pathways. Furthermore, combination of CX-4945 with other inhibitors yielded synergistic effects in cell death induction. These new findings demonstrate that CK2 overexpression contributes to blood cancer cell survival and resistance to chemotherapy. Combinatorial use of CX-4945 is a promising therapeutic tool for treatment of hematological malignancies.


