HyoscyamineCAS# 101-31-5 |
- chroman 1
Catalog No.:BCC1480
CAS No.:1273579-40-0
- Y-27632 dihydrochloride
Catalog No.:BCC1273
CAS No.:129830-38-2
- Hydroxyfasudil hydrochloride
Catalog No.:BCC1636
CAS No.:155558-32-0
- H-1152
Catalog No.:BCC1615
CAS No.:451462-58-1
- H-1152 dihydrochloride
Catalog No.:BCC1616
CAS No.:871543-07-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

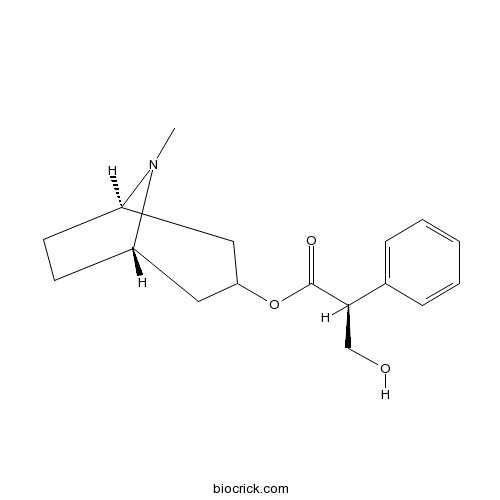
| Cas No. | 101-31-5 | SDF | Download SDF |
| PubChem ID | 154417 | Appearance | Powder |
| Formula | C17H23NO3 | M.Wt | 289.37 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Daturine | ||
| Solubility | DMSO : ≥ 36 mg/mL (124.41 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | [(1S,5R)-8-methyl-8-azabicyclo[3.2.1]octan-3-yl] (2S)-3-hydroxy-2-phenylpropanoate | ||
| SMILES | CN1C2CCC1CC(C2)OC(=O)C(CO)C3=CC=CC=C3 | ||
| Standard InChIKey | RKUNBYITZUJHSG-VFSICIBPSA-N | ||
| Standard InChI | InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15?,16-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Hyoscyamine is an AChR inhibitor with IC50 of 7.5 nM, it is widely used in medicine due to its anticholinergic activity. Hyoscyamine could be used to reduce abdominal discomfort and colonic spasm during a barium enema. |
| Targets | AChR |
| In vivo | Antispasmodic drugs to reduce discomfort and colonic spasm during barium enemas: comparison of oral hyoscyamine, i.v. glucagon, and no drug.[Pubmed: 8273637]AJR Am J Roentgenol. 1993 Nov;161(5):965-8.Parenterally administered glucagon is currently the agent of choice for reducing abdominal discomfort and colonic spasm during a barium enema. Because glucagon is expensive and frequently causes nausea, we evaluated the use of oral Hyoscyamine sulfate as an alternate agent and compared it with IV glucagon and no medication.
Use of atropine-diphenoxylate compared with hyoscyamine to decrease rates of irinotecan-related cholinergic syndrome.[Pubmed: 25839059]J Community Support Oncol. 2015 Jan;13(1):3-7.Cholinergic syndrome is a well established acute adverse reaction associated with irinotecan. Cholinergic side effects can be ameliorated or prevented with anticholinergic agents. To date, no formal studies have compared atropine-diphenoxylate and Hyoscyamine as premedications for prophylaxis of the cholinergic syndrome with irinotecan infusion.
To compare the incidence of cholinergic syndrome with irinotecan using atropine-diphenoxylate or Hyoscyamine as premedication.
|
| Structure Identification | Phytochem Anal. 2014 Jan-Feb;25(1):29-35.Multi-development-HPTLC method for quantitation of hyoscyamine, scopolamine and their biosynthetic precursors in selected solanaceae plants grown in natural conditions and as in vitro cultures.[Pubmed: 23839972]Hyoscyamine and scopolamine, anti-cholinergic agents widely used in medicine, are typically obtained from plants grown under natural conditions. Since field cultivation entails certain difficulties (changeable weather, pests, etc.), attempts have been made to develop a plant in vitro culture system as an alternative source for the production of these compounds. During experiments to locate the limiting steps in the biotechnological procedure, it is important to monitor not only the levels of the final products but also the changes in the concentration of their precursors.
To develop a HPTLC method for the separation and quantitation of the main tropane alkaloids Hyoscyamine and scopolamine, their respective direct precursors littorine and anisodamine, and cuscohygrine, a product of a parallel biosynthetic pathway that shares a common precursor (N-methyl-∆(1) -pyrrolium cation) with tropane alkaloids.
Microb Cell Fact. 2008 May 27;7:17.Expression of Brugmansia candida Hyoscyamine 6beta-Hydroxylase gene in Saccharomyces cerevisiae and its potential use as biocatalyst.[Pubmed: 18505565 ]Tropane alkaloids, mainly Hyoscyamine and scopolamine, are widely used in medicine due to their anticholinergic activity. Scopolamine has a higher demand being the more valuable alkaloid due to its fewer side effects and higher physiological activity. Anisodamine (6beta-hydroxyHyoscyamine) is the intermediate in the conversion of Hyoscyamine into scopolamine. Current studies report that this alkaloid is potentially applicable in medicine. The gene that codifies for Hyoscyamine 6-beta hydroxylase, the enzyme responsible for Hyoscyamine hydroxylation and epoxidation, leading to scopolamine was isolated from Brugmansia candida.
|

Hyoscyamine Dilution Calculator

Hyoscyamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4558 mL | 17.2789 mL | 34.5578 mL | 69.1157 mL | 86.3946 mL |
| 5 mM | 0.6912 mL | 3.4558 mL | 6.9116 mL | 13.8231 mL | 17.2789 mL |
| 10 mM | 0.3456 mL | 1.7279 mL | 3.4558 mL | 6.9116 mL | 8.6395 mL |
| 50 mM | 0.0691 mL | 0.3456 mL | 0.6912 mL | 1.3823 mL | 1.7279 mL |
| 100 mM | 0.0346 mL | 0.1728 mL | 0.3456 mL | 0.6912 mL | 0.8639 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Hyoscyamine is an AChR inhibitor with IC50 of 7.5 nM.
- Pyridostigmine Bromide
Catalog No.:BCC4579
CAS No.:101-26-8
- CY 208-243
Catalog No.:BCC6991
CAS No.:100999-26-6
- Levofloxacin
Catalog No.:BCC4791
CAS No.:100986-85-4
- CX-4945 (Silmitasertib)
Catalog No.:BCC3693
CAS No.:1009820-21-6
- NSC 687852 (b-AP15)
Catalog No.:BCC2389
CAS No.:1009817-63-3
- Thiamet G
Catalog No.:BCC4864
CAS No.:1009816-48-1
- Ebrotidine
Catalog No.:BCC1542
CAS No.:100981-43-9
- Rotundine
Catalog No.:BCN5983
CAS No.:10097-84-4
- 2,2-Bis(hydroxymethyl)butyric acid
Catalog No.:BCC8495
CAS No.:10097-02-6
- Stachyose
Catalog No.:BCN2566
CAS No.:10094-58-3
- Caulophyllumine A
Catalog No.:BCN7928
CAS No.:1009318-60-8
- AZD2014
Catalog No.:BCC3732
CAS No.:1009298-59-2
- Bis[4-(dimethylamino)phenyl]methane
Catalog No.:BCC8889
CAS No.:101-61-1
- MK-5108 (VX-689)
Catalog No.:BCC2176
CAS No.:1010085-13-8
- Microcystin-LR
Catalog No.:BCC5339
CAS No.:101043-37-2
- Larixinol
Catalog No.:BCN6484
CAS No.:101046-79-1
- Tenovin-3
Catalog No.:BCC3889
CAS No.:1011301-27-1
- 3,8'-Biapigenin
Catalog No.:BCN5825
CAS No.:101140-06-1
- Milnacipran HCl
Catalog No.:BCC4922
CAS No.:101152-94-7
- Odoriflavene
Catalog No.:BCN8240
CAS No.:101153-41-7
- Tenovin-6
Catalog No.:BCC3667
CAS No.:1011557-82-6
- Momordicoside P
Catalog No.:BCN3275
CAS No.:1011726-62-7
- Longipedlactone J
Catalog No.:BCN6644
CAS No.:1011762-93-8
- CUDC-101
Catalog No.:BCC2149
CAS No.:1012054-59-9
Use of atropine-diphenoxylate compared with hyoscyamine to decrease rates of irinotecan-related cholinergic syndrome.[Pubmed:25839059]
J Community Support Oncol. 2015 Jan;13(1):3-7.
BACKGROUND: Cholinergic syndrome is a well established acute adverse reaction associated with irinotecan. Cholinergic side effects can be ameliorated or prevented with anticholinergic agents. To date, no formal studies have compared atropine-diphenoxylate and Hyoscyamine as premedications for prophylaxis of the cholinergic syndrome with irinotecan infusion. OBJECTIVES: To compare the incidence of cholinergic syndrome with irinotecan using atropine-diphenoxylate or Hyoscyamine as premedication. METHODS: We conducted a retrospective, single-center, nonrandomized, cohort study of adult patients treated with atropine-diphenoxylate or Hyoscyamine as premedication before receiving irinotecan. For all irinotecan infusions, intravenous atropine was administered for patients experiencing any cholinergic reaction. RESULTS: A total of 532 irinotecan cycles (354 cycles for atropine-diphenoxylate group; 178 cycles for Hyoscyamine group) were analyzed in 80 patients. Overall incidence of cholinergic syndrome did not differ between atropine-diphenoxylate (8.2%) and Hyoscyamine (9.0%) groups (P = .76). The incidence of cholinergic syndrome after the poundrst cycle of irinotecan was similar between the 2 arms, atropine-diphenoxylate (14.6%) and Hyoscyamine (10.7%), with P = .74. The most common cholinergic symptoms documented were abdominal pain or cramping, and diarrhea. LIMITATIONS: This study was subjected to vulnerabilities to bias and random error because of its observational retrospective design and small number of participants. CONCLUSIONS: Lack of difference in the incidence of cholinergic syndrome observed in irinotecan-treated patients suggests atropinediphenoxylate and Hyoscyamine may both be effective prophylactic options. The findings support the need for a larger, randomized study to assess and compare these agents with other potential premedications such as scopolamine and atropine in prevention of irinotecan-related cholinergic syndrome.
Antispasmodic drugs to reduce discomfort and colonic spasm during barium enemas: comparison of oral hyoscyamine, i.v. glucagon, and no drug.[Pubmed:8273637]
AJR Am J Roentgenol. 1993 Nov;161(5):965-8.
OBJECTIVE: Parenterally administered glucagon is currently the agent of choice for reducing abdominal discomfort and colonic spasm during a barium enema. Because glucagon is expensive and frequently causes nausea, we evaluated the use of oral Hyoscyamine sulfate as an alternate agent and compared it with IV glucagon and no medication. SUBJECTS AND METHODS: A total of 349 adult patients undergoing barium enema examinations were randomly assigned in a prospective fashion to one of four groups: (1) no medication (87 patients); (2) 1 mg of IV glucagon (88 patients); (3) 0.125 mg of oral Hyoscyamine sulfate (87 patients); and (4) 0.25 mg of oral Hyoscyamine sulfate (87 patients). The degree of distension of the colon on radiographs obtained after fluoroscopy, the amount of abdominal distress after the procedure, and the number of side effects (nausea, palpitations, blurred vision, dry mouth) were analyzed. To evaluate the amount of abdominal discomfort more completely, we asked the last 248 patients to estimate the level of severity of the discomfort (none, mild, moderate, or severe). RESULTS: We found no difference in the degree of distension of the colon in the four groups (p = .63). Most patients (79%) had some degree of abdominal discomfort. Fewer patients in the group who received no medication (10%) had no or mild pain compared with those given glucagon (15%, p < .05), 0.125 mg of Hyoscyamine (14%, p < .05), or 0.25 mg of Hyoscyamine (12%, p = .15). Less nausea occurred in the group that received 0.125 mg of Hyoscyamine than in the other groups (p < .03). No patients in any of the groups had allergic or severe side effects. CONCLUSION: When compared with IV glucagon, oral Hyoscyamine had fewer side effects, but the degree of colonic distension or abdominal distress was not significantly different. In addition, Hyoscyamine is considerably less expensive than glucagon and can be given orally. Patients who received medications had less discomfort than those who did not. However, the degree of distension was not different.
Multi-development-HPTLC method for quantitation of hyoscyamine, scopolamine and their biosynthetic precursors in selected solanaceae plants grown in natural conditions and as in vitro cultures.[Pubmed:23839972]
Phytochem Anal. 2014 Jan-Feb;25(1):29-35.
INTRODUCTION: Hyoscyamine and scopolamine, anti-cholinergic agents widely used in medicine, are typically obtained from plants grown under natural conditions. Since field cultivation entails certain difficulties (changeable weather, pests, etc.), attempts have been made to develop a plant in vitro culture system as an alternative source for the production of these compounds. During experiments to locate the limiting steps in the biotechnological procedure, it is important to monitor not only the levels of the final products but also the changes in the concentration of their precursors. OBJECTIVE: To develop a HPTLC method for the separation and quantitation of the main tropane alkaloids Hyoscyamine and scopolamine, their respective direct precursors littorine and anisodamine, and cuscohygrine, a product of a parallel biosynthetic pathway that shares a common precursor (N-methyl-(1) -pyrrolium cation) with tropane alkaloids. METHODS: Using alkaloid extracts from Atropa baetica hairy roots, different TLC chromatographic systems and developing procedures were investigated. RESULTS: Full separation of all compounds was obtained on HPTLC Si60 F254 plates preconditioned with mobile phase vapours (chloroform:methanol:acetone:25% ammonia ratios of 75:15:10:1.8, v/v/v/v). The chromatograms were developed twice (at distances of 4.0 and 3.0 cm) in a Camag twin trough chamber and visualised with Dragendorff's reagent. Densitometric detection (lambda = 190 and 520 nm) was used for quantitative analyses of the different plant samples. CONCLUSION: This method can be recommended for quantitation of Hyoscyamine, scopolamine, anisodamine, littorine and cuscohygrine in different plant material (field grown vs. in vitro cultures).
Expression of Brugmansia candida Hyoscyamine 6beta-Hydroxylase gene in Saccharomyces cerevisiae and its potential use as biocatalyst.[Pubmed:18505565]
Microb Cell Fact. 2008 May 27;7:17.
BACKGROUND: Tropane alkaloids, mainly Hyoscyamine and scopolamine, are widely used in medicine due to their anticholinergic activity. Scopolamine has a higher demand being the more valuable alkaloid due to its fewer side effects and higher physiological activity. Anisodamine (6beta-hydroxyHyoscyamine) is the intermediate in the conversion of Hyoscyamine into scopolamine. Current studies report that this alkaloid is potentially applicable in medicine. The gene that codifies for Hyoscyamine 6-beta hydroxylase, the enzyme responsible for Hyoscyamine hydroxylation and epoxidation, leading to scopolamine was isolated from Brugmansia candida. RESULTS: The h6hcDNA was cloned into pYES2.1 and pYES2.1/V5-His-TOPO vectors to produce an untagged and a tagged protein, respectively. The H6H enzyme was produced in Saccharomyces cerevisiae in order to obtain a biological catalyst for potential industrial applications. Protein extracts of the induced yeast were analyzed by Western blot. The expression was detected 4 h after induction and no degradation was observed during the period assayed. The tagged and the untagged proteins were able to transform Hyoscyamine, showing a functional expression of the h6hcDNA. CONCLUSION: The strains obtained in this work are promising and potentially applicable in biocatalytic processes.


