NSC 687852 (b-AP15)19S regulatory particle Inhibitor CAS# 1009817-63-3 |
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1009817-63-3 | SDF | Download SDF |
| PubChem ID | 5351435 | Appearance | Powder |
| Formula | C22H17N3O6 | M.Wt | 419.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | b-AP15 | ||
| Solubility | DMSO : ≥ 44 mg/mL (104.91 mM) *"≥" means soluble, but saturation unknown. | ||
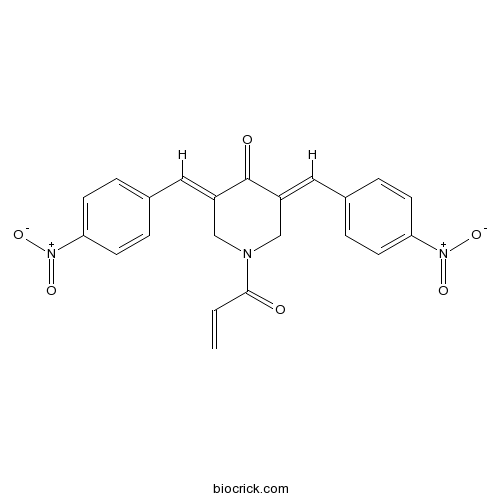
| Chemical Name | (3E,5E)-3,5-bis[(4-nitrophenyl)methylidene]-1-prop-2-enoylpiperidin-4-one | ||
| SMILES | C=CC(=O)N1CC(=CC2=CC=C(C=C2)[N+](=O)[O-])C(=O)C(=CC3=CC=C(C=C3)[N+](=O)[O-])C1 | ||
| Standard InChIKey | GFARQYQBWJLZMW-JYFOCSDGSA-N | ||
| Standard InChI | InChI=1S/C22H17N3O6/c1-2-21(26)23-13-17(11-15-3-7-19(8-4-15)24(28)29)22(27)18(14-23)12-16-5-9-20(10-6-16)25(30)31/h2-12H,1,13-14H2/b17-11+,18-12+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of 19S regulatory particle associated deubiquitinating enzymes (DUBs), UCHL5 and USP14. Shows minimal inhibition of UCHL-1, UCHL-3, USP2, USP7, USP, BAP1 and proteasome activity. Induces apoptosis by cathepsin D-dependent caspase-cleavage (IC50 = 0.5 μM). Displays antitumor activity in vivo. |

NSC 687852 (b-AP15) Dilution Calculator

NSC 687852 (b-AP15) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3844 mL | 11.9221 mL | 23.8442 mL | 47.6883 mL | 59.6104 mL |
| 5 mM | 0.4769 mL | 2.3844 mL | 4.7688 mL | 9.5377 mL | 11.9221 mL |
| 10 mM | 0.2384 mL | 1.1922 mL | 2.3844 mL | 4.7688 mL | 5.961 mL |
| 50 mM | 0.0477 mL | 0.2384 mL | 0.4769 mL | 0.9538 mL | 1.1922 mL |
| 100 mM | 0.0238 mL | 0.1192 mL | 0.2384 mL | 0.4769 mL | 0.5961 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: N/A
NSC 687852 is a 19S regulatory particle inhibitor.
The 19S particles bind polyubiquitin-linked polypeptides and present them to the 20S degradative units. USP14 and UCHL5 are cysteine enzymes that become activated after being associated with the proteasome.
In vitro: NSC 687852 blocked deubiquitylating activity of USP14 and UCHL5 selectively without inhibiting proteasome activity. NSC 687852 decreased viability in multiple myeloma (MM) cell lines and patient MM cells, inhibited MM cell proliferation even in the presence of bone marrow stroma cells, and overcomed bortezomib resistance. Anti-MM activity of NSC 687852 was associated with growth arrest through downregulating CDC2, CDC25C, and cyclin B1, as well as induction of caspase-dependent apoptosis and activation of unfolded protein response [1].
In vivo: In vivo studies using distinct human MM xenograft models showed that NSC 687852 was well tolerated, inhibited tumor growth, and prolonged mouse survival. Combination of NSC 687852 with suberoylanilide hydroxamic acid, lenalidomide, or dexamethasone was found to induce synergistic anti-MM activity [1].
Clinical trial: N/A
Reference:
[1] Ze Tian,Padraig D'Arcy,Xin Wang,Arghya Ray,Yu-Tzu Tai,Yiguo Hu,Ruben D Carrasco,Paul Richardson,Stig Linder,Dharminder Chauhan,Kenneth C Anderson. A novel small molecule inhibitor of deubiquitylating enzyme USP14 and UCHL5 induces apoptosis in multiple myeloma and overcomes bortezomib resistance. Blood. 2014 Jan 30; 123(5): 706–716.
- Thiamet G
Catalog No.:BCC4864
CAS No.:1009816-48-1
- Ebrotidine
Catalog No.:BCC1542
CAS No.:100981-43-9
- Rotundine
Catalog No.:BCN5983
CAS No.:10097-84-4
- 2,2-Bis(hydroxymethyl)butyric acid
Catalog No.:BCC8495
CAS No.:10097-02-6
- Stachyose
Catalog No.:BCN2566
CAS No.:10094-58-3
- Caulophyllumine A
Catalog No.:BCN7928
CAS No.:1009318-60-8
- AZD2014
Catalog No.:BCC3732
CAS No.:1009298-59-2
- AZD8055
Catalog No.:BCC3629
CAS No.:1009298-09-2
- Panamycin 607
Catalog No.:BCN1813
CAS No.:100905-89-3
- Piceatannol
Catalog No.:BCN5824
CAS No.:10083-24-6
- (RS)-CPP
Catalog No.:BCC6561
CAS No.:100828-16-8
- 4,4'-Bis(α,α-dimethylbenzyl)diphenylamine
Catalog No.:BCC8661
CAS No.:10081-67-1
- CX-4945 (Silmitasertib)
Catalog No.:BCC3693
CAS No.:1009820-21-6
- Levofloxacin
Catalog No.:BCC4791
CAS No.:100986-85-4
- CY 208-243
Catalog No.:BCC6991
CAS No.:100999-26-6
- Pyridostigmine Bromide
Catalog No.:BCC4579
CAS No.:101-26-8
- Hyoscyamine
Catalog No.:BCN1946
CAS No.:101-31-5
- Bis[4-(dimethylamino)phenyl]methane
Catalog No.:BCC8889
CAS No.:101-61-1
- MK-5108 (VX-689)
Catalog No.:BCC2176
CAS No.:1010085-13-8
- Microcystin-LR
Catalog No.:BCC5339
CAS No.:101043-37-2
- Larixinol
Catalog No.:BCN6484
CAS No.:101046-79-1
- Tenovin-3
Catalog No.:BCC3889
CAS No.:1011301-27-1
- 3,8'-Biapigenin
Catalog No.:BCN5825
CAS No.:101140-06-1
- Milnacipran HCl
Catalog No.:BCC4922
CAS No.:101152-94-7
Inhibition of proteasome deubiquitinating activity as a new cancer therapy.[Pubmed:22057347]
Nat Med. 2011 Nov 6;17(12):1636-40.
Ubiquitin-tagged substrates are degraded by the 26S proteasome, which is a multisubunit complex comprising a proteolytic 20S core particle capped by 19S regulatory particles. The approval of bortezomib for the treatment of multiple myeloma validated the 20S core particle as an anticancer drug target. Here we describe the small molecule b-AP15 as a previously unidentified class of proteasome inhibitor that abrogates the deubiquitinating activity of the 19S regulatory particle. b-AP15 inhibited the activity of two 19S regulatory-particle-associated deubiquitinases, ubiquitin C-terminal hydrolase 5 (UCHL5) and ubiquitin-specific peptidase 14 (USP14), resulting in accumulation of polyubiquitin. b-AP15 induced tumor cell apoptosis that was insensitive to TP53 status and overexpression of the apoptosis inhibitor BCL2. We show that treatment with b-AP15 inhibited tumor progression in four different in vivo solid tumor models and inhibited organ infiltration in an acute myeloid leukemia model. Our results show that the deubiquitinating activity of the 19S regulatory particle is a new anticancer drug target.
Induction of the lysosomal apoptosis pathway by inhibitors of the ubiquitin-proteasome system.[Pubmed:19089926]
Int J Cancer. 2009 Mar 15;124(6):1463-9.
The lysosomal apoptosis pathway is a potentially interesting therapeutic target. Since apoptosis involving the lysosomal pathway has been described to involve cathepsins, we screened a drug library for agents that induce cathepsin-dependent apoptosis. Using pharmacological inhibitors and siRNA, we identified 2 structurally related agents (NSC687852 and NSC638646) that induced cathepsin D-dependent caspase-cleavage activity in human breast cancer cells. Both agents were found to induce the mitochondrial apoptosis pathway. NSC687852 and NSC638646 were found to inhibit the activity of ubiquitin isopeptidases and to induce the accumulation of high-molecular-mass ubiquitins in cells. We show that 3 other inhibitors of the proteasome degradation pathway induce lysosomal membrane permeabilization (LMP) and that cathepsin-D siRNA inhibits apoptosis induced by these agents. We conclude that a screen for cathepsin-dependent apoptosis-inducing agents resulted in the identification of ubiquitin isopeptidase inhibitors and that proteasome inhibitors with different mechanisms of action induce LMP and cathepsin D-dependent apoptosis.


