Milnacipran HCl5-HT and noradrenalin re-uptake inhibitor (SNRI) CAS# 101152-94-7 |
- Nepicastat (SYN-117) HCl
Catalog No.:BCC2286
CAS No.:170151-24-3
- Isotretinoin
Catalog No.:BCC2284
CAS No.:4759-48-2
- Disulfiram
Catalog No.:BCC2098
CAS No.:97-77-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 101152-94-7 | SDF | Download SDF |
| PubChem ID | 163701 | Appearance | Powder |
| Formula | C15H23ClN2O | M.Wt | 282.81 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 100 mg/mL (353.59 mM) DMSO : ≥ 48 mg/mL (169.73 mM) *"≥" means soluble, but saturation unknown. | ||
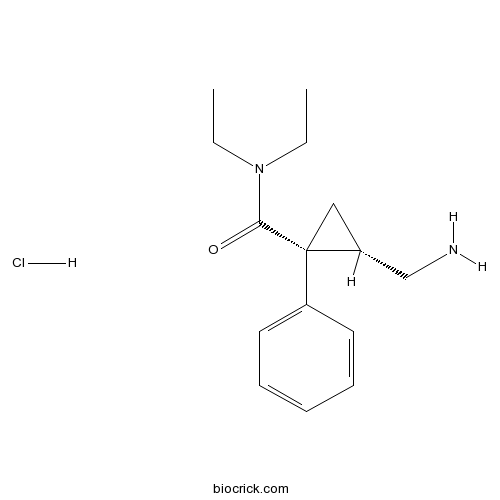
| Chemical Name | (1R*,2S*)-2-(Aminomethyl)-N,N-dieth | ||
| SMILES | [Cl-].CCN(CC)C(=O)[C@@]1(C[C@@H]1CN)c2ccccc2.[H+] | ||
| Standard InChIKey | XNCDYJFPRPDERF-PBCQUBLHSA-N | ||
| Standard InChI | InChI=1S/C15H22N2O.ClH/c1-3-17(4-2)14(18)15(10-13(15)11-16)12-8-6-5-7-9-12;/h5-9,13H,3-4,10-11,16H2,1-2H3;1H/t13-,15+;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Orally active 5-HT and noradrenalin re-uptake inhibitor (SNRI) (IC50 values are 203 and 100 nM respectively) that displays no affinity at a range of other receptors. Causes adaptive changes to α1-adrenergic and 5-HT2A serotonergic systems when administered repeatedly. Exhibits antidepressive and antinociceptive activities in vivo. |

Milnacipran HCl Dilution Calculator

Milnacipran HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5359 mL | 17.6797 mL | 35.3594 mL | 70.7189 mL | 88.3986 mL |
| 5 mM | 0.7072 mL | 3.5359 mL | 7.0719 mL | 14.1438 mL | 17.6797 mL |
| 10 mM | 0.3536 mL | 1.768 mL | 3.5359 mL | 7.0719 mL | 8.8399 mL |
| 50 mM | 0.0707 mL | 0.3536 mL | 0.7072 mL | 1.4144 mL | 1.768 mL |
| 100 mM | 0.0354 mL | 0.1768 mL | 0.3536 mL | 0.7072 mL | 0.884 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Milnacipran hydrochloride is a serotonin-norepinephrine reuptake inhibitor (SNRI) used in the clinical treatment of fibromyalgia.
- 3,8'-Biapigenin
Catalog No.:BCN5825
CAS No.:101140-06-1
- Tenovin-3
Catalog No.:BCC3889
CAS No.:1011301-27-1
- Larixinol
Catalog No.:BCN6484
CAS No.:101046-79-1
- Microcystin-LR
Catalog No.:BCC5339
CAS No.:101043-37-2
- MK-5108 (VX-689)
Catalog No.:BCC2176
CAS No.:1010085-13-8
- Bis[4-(dimethylamino)phenyl]methane
Catalog No.:BCC8889
CAS No.:101-61-1
- Hyoscyamine
Catalog No.:BCN1946
CAS No.:101-31-5
- Pyridostigmine Bromide
Catalog No.:BCC4579
CAS No.:101-26-8
- CY 208-243
Catalog No.:BCC6991
CAS No.:100999-26-6
- Levofloxacin
Catalog No.:BCC4791
CAS No.:100986-85-4
- CX-4945 (Silmitasertib)
Catalog No.:BCC3693
CAS No.:1009820-21-6
- NSC 687852 (b-AP15)
Catalog No.:BCC2389
CAS No.:1009817-63-3
- Odoriflavene
Catalog No.:BCN8240
CAS No.:101153-41-7
- Tenovin-6
Catalog No.:BCC3667
CAS No.:1011557-82-6
- Momordicoside P
Catalog No.:BCN3275
CAS No.:1011726-62-7
- Longipedlactone J
Catalog No.:BCN6644
CAS No.:1011762-93-8
- CUDC-101
Catalog No.:BCC2149
CAS No.:1012054-59-9
- IRAK inhibitor 4
Catalog No.:BCC1657
CAS No.:1012104-68-5
- Picrasidine Q
Catalog No.:BCN3182
CAS No.:101219-61-8
- IRAK inhibitor 3
Catalog No.:BCC1656
CAS No.:1012343-93-9
- Kushenol K
Catalog No.:BCN3448
CAS No.:101236-49-1
- Kushenol L
Catalog No.:BCN3309
CAS No.:101236-50-4
- Kushenol M
Catalog No.:BCN3310
CAS No.:101236-51-5
- Phenserine
Catalog No.:BCC7529
CAS No.:101246-66-6
Innovation of novel 'Tab in Tab' system for release modulation of milnacipran HCl: optimization, formulation and in vitro investigations.[Pubmed:23210688]
Drug Dev Ind Pharm. 2013 Nov;39(11):1851-63.
The study was aimed toward development of modified release oral drug delivery system for highly water soluble drug, Milnacipran HCl (MH). Novel Tablet in Tablet system (TITs) comprising immediate and extended release dose of MH in different parts was fabricated. The outer shell was composed of admixture of MH, lactose and novel herbal disintegrant obtained from seeds of Lepidium sativum. In the inner core, MH was matrixed with blend of hydrophilic (Benecel(R)) and hydrophobic (Compritol(R)) polymers. 3(2) full factorial design and an artificial neuron network (ANN) were employed for correlating effect of independent variables on dependent variables. The TITs were characterized for pharmacopoeial specifications, in vitro drug release, SEM, drug release kinetics and FTIR study. The release pattern of MH from batch A10 containing 25.17% w/w Benecel(R) and 8.21% w/w of Compritol(R) exhibited drug release pattern close proximal to the ideal theoretical profile (t(50%) = 5.92 h, t(75%) = 11.9 h, t(90%) = 18.11 h). The phenomenon of drug release was further explained by concept of percolation and the role of Benecel(R) and Compritol(R) in drug release retardation was studied. The normalized error obtained from ANN was less, compared with the multiple regression analysis, and exhibits the higher accuracy in prediction. The results of short-term stability study revealed stable chataracteristics of TITs. SEM study of TITs at different dissolution time points confirmed both diffusion and erosion mechanisms to be operative during drug release from the batch A10. Novel TITs can be a succesful once a day delivery system for highly water soluble drugs.
In vivo performance evaluation and establishment of IVIVC for osmotic pump based extended release formulation of milnacipran HCl.[Pubmed:23463628]
Biopharm Drug Dispos. 2013 May;34(4):227-35.
The objective of the present study was to carry out a pharmacokinetics evaluation of an oral modified release formulation [Aquarius EKX 19102 SRX-2 based osmotic pump (OP)] containing highly soluble Milnacipran HCl (MH) as a model drug. It was also aimed at developing an in vitro-in vivo correlation (IVIVC) model for a developed OP. In vivo plasma concentration data were obtained from six healthy male New Zealand albino rabbits after administration of immediate-release Milnacipran HCl solution (IRMHSOL) and Milnacipran HCl osmotic pump (MHOP). In vitro samples were analysed using an in house developed spectrophotometry method and in vivo samples were analysed using a RP-HPLC method developed by the author. A deconvolution based Level A model was attempted through a correlation of the percent in vivo input obtained through deconvolution and the percent in vitro dissolution obtained experimentally. A good correlation between the percentages dissolved vs absorbed (R(2) = 0.978) was obtained using level A correlation. Evaluation of the internal predictability of level A correlation was calculated in terms of the percent prediction error, which was found to be below 15%. In a nutshell, the success of the present study warrants further studies in patient volunteers to assess the ability of the MHOP to provide an effective therapy for depression.
Antiallodynic and antihyperalgesic effect of milnacipran in mice with spinal nerve ligation.[Pubmed:18349211]
Anesth Analg. 2008 Apr;106(4):1309-15, table of contents.
BACKGROUND: The antidepressant, milnacipran, has been reported to have antinociceptive, antiallodynic, and antihyperalgesic effects. In this study, we examined the mechanisms of the antiallodynic and antihyperalgesic effects of milnacipran in a model of neuropathic pain induced by spinal nerve ligation in mice. METHODS: The fifth left lumbar nerve of male C57BL6 mice was tightly ligated. Withdrawal threshold to tactile stimulation and withdrawal latency to heat stimulation in the injured or contralateral paw was tested by using von Frey filaments and radiant heat, respectively. RESULTS: Milnacipran was administered either orally (7.5-120 mg/kg), intrathecally, intracerebroventricularly, or locally (210 ng-21 microg). Both systemic, intrathecal and intracerebroventricular milnacipran increased withdrawal threshold and withdrawal latency in nerve-ligated mice whereas local injection had no effect. Depletion of spinal serotonergic or noradrenergic neurons was achieved by use of the specific neurotoxins, 6-hydroxydopamine or 5,7-dihydroxytryptamine, applied intrathecally 3 days before evaluation of the analgesic effect of milnacipran. Spinal serotonergic and noradrenergic denervation attenuated the effect of milnacipran in sham-operated mice. In nerve-ligated mice, however, the effect of milnacipran was lost after noradrenergic denervation but not after serotonergic denervation. CONCLUSIONS: We concluded that the antiallodynic and antihyperalgesic effects of milnacipran on neuropathic pain induced by spinal nerve ligation are principally mediated through action at supraspinal and spinal sites via activation of the spinal noradrenergic system.
Pharmacological effects of milnacipran, a new antidepressant, given repeatedly on the alpha1-adrenergic and serotonergic 5-HT2A systems.[Pubmed:11145008]
J Neural Transm (Vienna). 2000;107(11):1345-59.
Milanacipran (MIL) is a representative of a new class of antidepressants (SNRIs) which inhibit selectively the reuptake of serotonin and noradrenaline but, in contrast to tricyclics, show no affinity for neurotransmitter receptors. The present study was aimed at determining whether repeated MIL treatment induced adaptive changes in the alpha1-adrenergic and serotonergic 5-HT2A systems, similar to those reported by us earlier for tricyclic antidepressants. The experiments were carried out on male mice and rats. MIL was administered at a dose of 10 or 30 mg/kg p.o. once or repeatedly (twice daily for 14 days). The obtained results showed that MIL administered repeatedly potentiated the clonidine-induced aggressiveness and the methoxamine-induced exploratory hyperactivity, the effects mediated by alpha1-adrenoceptors. MIL did not change the number or affinity (Bmax and K(D)) of alpha1-adrenergic receptors in the cerebral cortex for [3H]prazonsin, however, the ability of the alpha1-adrenoceptor agonist phenylephrine to compete for these sites was significantly enhanced. MIL given repeatedly (but not acutely) inhibited both the head twitch reaction induced by L-5-HTP or (+/-)DOI, the effects mediated by serotonergic 5-HT2A receptors. MIL also decreased the binding (Bmax) or [3H]-ketanserin to 5-HT2A receptors in the cerebral cortex. The above results indicate that repeated MIL administration increases the responsiveness of alpha1-adrenergic system (behavioural and biochemical changes) and decreases the responsiveness of the serotonergic 5-HT2A receptors (especially behavioural changes) as tricyclics do. It may be concluded that the lack of MIL affinity for neurotransmitter receptors is of no importance to the development of adaptive changes in the studied systems, observed after repeated treatment with antidepressants.
Biochemical profile of midalcipran (F 2207), 1-phenyl-1-diethyl-aminocarbonyl-2-aminomethyl-cyclopropane (Z) hydrochloride, a potential fourth generation antidepressant drug.[Pubmed:3005901]
Neuropharmacology. 1985 Dec;24(12):1211-9.
The present study of midalcipran (F 2207), 1-phenyl-1-diethyl-aminocarbonyl-2-aminomethyl-cyclopropane(Z) hydrochloride, was undertaken to determine its biochemical profile. The properties of midalcipran, in inhibiting the uptake of monoamines were tested and compared with that of imipramine. In vitro, midalcipran was found to inhibit the uptake of radiolabelled serotonin and noradrenaline (IC50 = 203 and 100 nM, respectively), but not that of dopamine, into brain slices. Hyperthermia induced by the centrally-acting displacers of monoamines, H77/77 and H75/12, were almost equipotently antagonized by midalcipran, confirming the inhibition of the uptake of serotonin and noradrenaline by midalcipran in vivo (ID50 = 11 and 4.8 mg/kg, respectively). Midalcipran showed no inhibition of the activity of monoamine oxidase in vitro or in vivo. The interaction between midalcipran and neurotransmitter receptors and binding sites in the CNS was studied in the rat in comparison with imipramine and desipramine. In contrast to these two antidepressant drugs, midalcipran showed no affinity for alpha- or beta-adrenoceptors, muscarinic, histaminergic H1, dopaminergic D2 or serotonergic 5-HT2 receptors, suggesting a general absence of anticholinergic, sedative and other side-effects. Midalcipran was equipotent with imipramine at inhibiting the binding of [3H]imipramine. Chronic administration of midalcipran to rats did not alter the number of beta-adrenergic receptors in the cortex, in contrast to imipramine and desipramine which decreased the binding of beta-adrenoceptors. Thus midalcipran appears to act exclusively presynaptically, inhibiting the uptake of serotonin and noradrenaline. This activity, coupled to the total absence of interaction at postsynaptic sites, suggests that midalcipran may be a useful and novel antidepressant drug.


