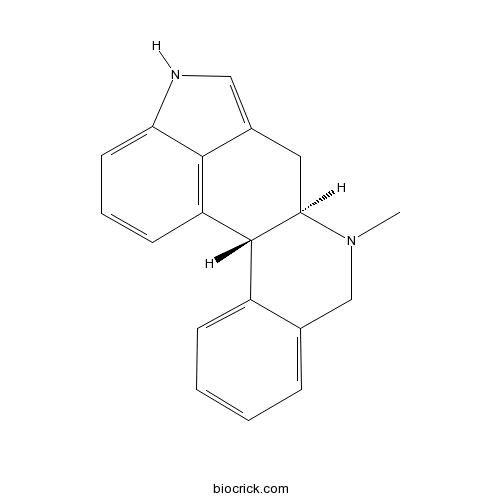
CY 208-243Selective D1-like agonist CAS# 100999-26-6 |
- SGI-1776 free base
Catalog No.:BCC2232
CAS No.:1025065-69-3
- LKB1 (AAK1 dual inhibitor)
Catalog No.:BCC1705
CAS No.:1093222-27-5
- CX-6258
Catalog No.:BCC1504
CAS No.:1202916-90-2
- AZD1208
Catalog No.:BCC2079
CAS No.:1204144-28-4
- SMI-4a
Catalog No.:BCC2233
CAS No.:438190-29-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 100999-26-6 | SDF | Download SDF |
| PubChem ID | 58144 | Appearance | Powder |
| Formula | C19H18N2 | M.Wt | 274.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
| SMILES | CN1CC2=CC=CC=C2C3C1CC4=CNC5=CC=CC3=C45 | ||
| Standard InChIKey | WRNKIDLXXXIELU-IEBWSBKVSA-N | ||
| Standard InChI | InChI=1S/C19H18N2/c1-21-11-12-5-2-3-6-14(12)19-15-7-4-8-16-18(15)13(10-20-16)9-17(19)21/h2-8,10,17,19-20H,9,11H2,1H3/t17-,19-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Centrally active dopamine D1 receptor agonist, selective over D2 receptor sites. Stimulates adenylate cyclase in rat striatal homogenates with an EC50 of 125 nM. Unlike SKF 38393, it exerts antiParkinsonian activity in animal models. |

CY 208-243 Dilution Calculator

CY 208-243 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6448 mL | 18.2242 mL | 36.4485 mL | 72.8969 mL | 91.1212 mL |
| 5 mM | 0.729 mL | 3.6448 mL | 7.2897 mL | 14.5794 mL | 18.2242 mL |
| 10 mM | 0.3645 mL | 1.8224 mL | 3.6448 mL | 7.2897 mL | 9.1121 mL |
| 50 mM | 0.0729 mL | 0.3645 mL | 0.729 mL | 1.4579 mL | 1.8224 mL |
| 100 mM | 0.0364 mL | 0.1822 mL | 0.3645 mL | 0.729 mL | 0.9112 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Levofloxacin
Catalog No.:BCC4791
CAS No.:100986-85-4
- CX-4945 (Silmitasertib)
Catalog No.:BCC3693
CAS No.:1009820-21-6
- NSC 687852 (b-AP15)
Catalog No.:BCC2389
CAS No.:1009817-63-3
- Thiamet G
Catalog No.:BCC4864
CAS No.:1009816-48-1
- Ebrotidine
Catalog No.:BCC1542
CAS No.:100981-43-9
- Rotundine
Catalog No.:BCN5983
CAS No.:10097-84-4
- 2,2-Bis(hydroxymethyl)butyric acid
Catalog No.:BCC8495
CAS No.:10097-02-6
- Stachyose
Catalog No.:BCN2566
CAS No.:10094-58-3
- Caulophyllumine A
Catalog No.:BCN7928
CAS No.:1009318-60-8
- AZD2014
Catalog No.:BCC3732
CAS No.:1009298-59-2
- AZD8055
Catalog No.:BCC3629
CAS No.:1009298-09-2
- Panamycin 607
Catalog No.:BCN1813
CAS No.:100905-89-3
- Pyridostigmine Bromide
Catalog No.:BCC4579
CAS No.:101-26-8
- Hyoscyamine
Catalog No.:BCN1946
CAS No.:101-31-5
- Bis[4-(dimethylamino)phenyl]methane
Catalog No.:BCC8889
CAS No.:101-61-1
- MK-5108 (VX-689)
Catalog No.:BCC2176
CAS No.:1010085-13-8
- Microcystin-LR
Catalog No.:BCC5339
CAS No.:101043-37-2
- Larixinol
Catalog No.:BCN6484
CAS No.:101046-79-1
- Tenovin-3
Catalog No.:BCC3889
CAS No.:1011301-27-1
- 3,8'-Biapigenin
Catalog No.:BCN5825
CAS No.:101140-06-1
- Milnacipran HCl
Catalog No.:BCC4922
CAS No.:101152-94-7
- Odoriflavene
Catalog No.:BCN8240
CAS No.:101153-41-7
- Tenovin-6
Catalog No.:BCC3667
CAS No.:1011557-82-6
- Momordicoside P
Catalog No.:BCN3275
CAS No.:1011726-62-7
Structure-activity relationships in the trans-hexahydroindolo[4,3-ab]phenanthridine ("benzergoline") series. 2. Resolution, absolute configuration, and dopaminergic activity of the selective D1 agonist CY 208-243 and its implication for an "extended rotamer-based dopamine receptor model".[Pubmed:8097540]
J Med Chem. 1993 Apr 16;36(8):977-84.
4,6,6a,7,8,12b-Hexahydroindolo[4,3-ab]phenanthridines ("benzergolines") was the first structural class of potent and selective dopamine D1 agonists lacking a catechol group. In order to determine the enantioselectivity of the 7-methyl derivative in the adenylate cyclase assay, its 5,5a-dihydro precursor was resolved and both enantiomers oxidized to the final products. The biological activity was found to reside entirely in the (-)-enantiomer, (-)-1 (CY 208-243). An X-ray study of its (-)-mandelic acid salt revealed a 6aR,12bR absolute configuration, which, in confirmation of the structure hypothesis, corresponds to that of the ergolines. Unexpectedly, an axial conformation of the N-methyl group was observed in the crystal structure. In contrast, subsequently analyzed crystals of the free base of (-)-1 revealed an equatorial conformation of the N-methyl group, which, we assume, represents the bioactive conformation. Based on the determined absolute configuration, (-)-1 could be oriented in a previously described "rotamer-based dopamine receptor model", which allowed the localization of a "subtype selectivity-inducing site" (aryl binding site at the D1 receptor, steric barrier at the D2 receptor), marked by the conformationally fixed "additional" phenyl group of the benzergoline molecule.
Dopamine agonists suppress cholinomimetic-induced tremulous jaw movements in an animal model of Parkinsonism: tremorolytic effects of pergolide, ropinirole and CY 208-243.[Pubmed:15582103]
Behav Brain Res. 2005 Jan 30;156(2):173-9.
Considerable evidence indicates that cholinomimetic-induced tremulous jaw movements in rats share many characteristics with human Parkinsonian tremor, and several antiparkinsonian drugs suppress cholinomimetic-induced tremulous jaw movements. The present study investigated three different types of dopamine agonists, which have known antiparkinsonian characteristics, for their ability to suppress the tremulous jaw movements induced by tacrine (5.0 mg/kg). The non-selective dopamine agonist pergolide, a widely used antiparkinsonian drug, was highly potent at suppressing tacrine-induced jaw movements (e.g. 0.125-1.0 mg/kg). The selective D2 agonist ropinirole, which also is used clinically as an antiparkinsonian drug, suppressed jaw movements in the dose range of 2.5-20.0 mg/kg. The D1 agonist CY 208-243, which has been reported to suppress tremor, also reduced jaw movement activity (4.0 mg/kg). Across several studies, the rank order of potency for suppressing cholinomimetic-induced jaw movements in rats is related to the potency for producing antiparkinsonian effects in humans. Together with previous studies, the present results suggest that cholinomimetic-induced jaw movements in rats can be used to characterize dopaminergic antiparkinsonian agents and to investigate the basal ganglia circuits involved in the generation of tremulous movements.
Effect of adding the D-1 agonist CY 208-243 to chronic bromocriptine treatment of MPTP-monkeys: regional changes of brain dopamine receptors.[Pubmed:8588064]
Prog Neuropsychopharmacol Biol Psychiatry. 1995 Jul;19(4):667-76.
1. Eleven monkeys were administered N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): eight were treated with bromocriptine for one week and then CY 208-243 (four monkeys) or saline (four monkeys) was added to the bromocriptine treatment. 2. Addition of CY 208-243 increased the therapeutic response observed with the ergot alone without inducing dyskinesia. 3. Following MPTP, [3H]-SCH 23390 specific binding to D-1 receptors as well as [3H]-spiperone and [3H]-N-n-propylnorapomorphine specific binding to D-2 receptors increased in posterior striatum compared to control animals, whereas [3H]-SKF 38393 binding to D-1 receptors tended to decrease. 4. Dopamine receptor density was unchanged in anterior striatum of untreated MPTP-monkeys. 5. In the posterior striatum, both dopaminergic treatments decreased towards control values [3H]-SCH 23390, [3H]-spiperone and [3H]-N-n-propylnorapomorphine binding whereas they did not significantly change [3H]-SKF 38393 specific binding. [3H]-SKF 38393 specific binding increased in anterior striatum of bromocriptine-treated MPTP-monkeys, compared to untreated MPTP-animals, and this increase was abolished in animals treated with bromocriptine+CY 208-243. 6. The present study shows that in MPTP-monkeys, treated or not with DA agonists, the D1 and D2 receptor changes are concentrated in the posterior striatum and that denervation appears to cause a shift from the high to the low affinity agonist state of D1 receptors but not for the D2 subtype.
Chronic CY 208-243 treatment of MPTP-monkeys causes regional changes of dopamine and GABAA receptors.[Pubmed:7905197]
Neurosci Lett. 1993 Nov 26;163(1):31-5.
Four monkeys were rendered parkinsonian by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) i.v. administration and then treated chronically with increasing doses of the D1 agonist CY 208-243 (0.05, 0.1 and 0.5 mg/kg). All animals showed a dose-dependent improvement of their parkinsonian signs after the chronic CY 208-243 treatment; however, half of them developed peak-dose dyskinesias. Dopamine levels were more decreased in the striatum of MPTP-monkeys with dyskinesias compared to those without dyskinesias. [3H]SCH 23390 and [3H]SKF 38393 binding to D1 receptors were in general similar in the striatum of both groups of MPTP-monkeys except [3H]SKF 38393 binding which was lower in the posterior putamen of dyskinetic compared to non-dyskinetic monkeys reflecting decreased coupling of this receptor to G proteins. [3H]spiperone and [3H]N-n-propylnorapomorphine binding to D2 receptors in the striatum tended in general to be higher in dyskinetic compared to non-dyskinetic monkeys, and this reached statistical significance in the posterior caudate labelled with [3H]n-propylnorapomorphine. [3H]muscimol binding to GABAA receptors was significantly higher in the posterior caudate of dyskinetic compared to non-dyskinetic monkeys. The extent of striatal DA denervation, decreased D1, elevated D2 and GABAA receptors, as well as the decrease of the D1/D2 receptor ratio in the posterior striatum may be involved in the appearance of dyskinesias after chronic CY 208-243 treatment.
Dopamine receptor agonists: selectivity and dopamine D1 receptor efficacy.[Pubmed:1973652]
Eur J Pharmacol. 1990 Jun 12;188(6):335-47.
Dopamine receptor selectivity was investigated for a number of dopamine receptor agonists. In vitro, the benzazepine derivatives, e.g., SKF 38393 and SKF 75670 as well as the isoquinoline derivatives, SKF 89626 and SKF 89615, were D1 receptor-selective. All other compounds like apomorphine, CY 208-243, 6,7-ADTN and 3-PPP were either D2-selective or did not discriminate between subtypes. In general, the same receptor profile seen in vitro was observed in vivo. The exceptions to this pattern were: compounds which did not cross the blood-brain barrier, like 6,7-ADTN and SKF 89626, and compounds which appeared nonselective in vitro but demonstrated D2 selectivity in vivo like apomorphine, CI 201-678 and CY 208-243. A number of compounds were characterized in detail with respect to a GTP-induced affinity shift in inhibition of [3H]SCH 23390 binding, and potency and efficacy in stimulating adenylate cyclase from rat striatum. Inhibition of specific [3H]SCH 23390 binding by these agonists in the absence of GTP occurred with Hill slopes below unity and could best be explained by a two-site model with a high (KH)- and low-affinity (KL) component. Inhibition of [3H]SCH 23390 binding in the presence of 15 microM GTP occurred with Hill slopes of unity. The KI values obtained in the presence of 15 microM GTP were similar to the KL values, the low-affinity component observed in the absence of GTP. The capability of the agonists to stimulate the adenylate cyclase was analyzed in relation to dopamine (efficacy = 100%). The efficacy of the benzazepine derivatives varied from 24 (SKF 75670) to 100% (SKF 83189), dependent on the substituents on the benzazepine core. The isoquinolines, SKF 89626 and SKF 89615 had full efficacy, whereas most other agonists tested appeared to have only partial efficacy. In summary, the present paper presents data on dopamine receptor selectivity and efficacy in stimulating adenylate cyclase for a number of dopaminergic agonists. These data may create a basis for selection of agonists in future characterizations of dopaminergic-mediated events.
Antiparkinsonian activity of CY 208-243, a partial D-1 dopamine receptor agonist, in MPTP-treated marmosets and patients with Parkinson's disease.[Pubmed:2571082]
Mov Disord. 1989;4(3):261-5.
The effect of stimulation of cerebral dopamine D-1 receptors by CY 208-243 on motor disability was tested in MPTP-treated parkinsonian marmosets and patients with Parkinson's disease. CY 208-243 (0.5-1.25 mg/kg s.c.) produced a dose-related reversal of akinesia and rigidity in the marmosets, lasting some 2 h. Single morning doses of CY 208-243 (5-40 mg) were compared with the usual morning dose of levodopa in eight patients with Parkinson's disease on long-term levodopa therapy who had developed motor fluctuations from immobility with akinesia and rigidity (off) to mobility often with dyskinesias (on). CY 208-243 alone was capable of switching such patients from off to on; five of the eight patients responded to the highest dose (40 mg), sometimes with dyskinesias. The response to CY 208-243 was comparable to that produced by levodopa in these cases. Drugs designed to stimulate both dopamine D1 and D2 receptors in the brain may improve the therapy of Parkinson's disease.


