Microcystin-LRInhibitor of protein phosphatase PP1/PP2A,potent and selective CAS# 101043-37-2 |
- Mibefradil dihydrochloride
Catalog No.:BCC1749
CAS No.:116666-63-8
- Cilnidipine
Catalog No.:BCC1083
CAS No.:132203-70-4
- Pregabalin
Catalog No.:BCN2175
CAS No.:148553-50-8
- NNC 55-0396
Catalog No.:BCC1803
CAS No.:357400-13-6
- NP118809
Catalog No.:BCC1807
CAS No.:41332-24-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 101043-37-2 | SDF | Download SDF |
| PubChem ID | 445434 | Appearance | Powder |
| Formula | C49H74N10O12 | M.Wt | 995.17 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Cyanoginosin-LR; MC-LR; Toxin T 17 (Microcystis aeruginosa) | ||
| Solubility | Ethanol : 0.5 mg/mL (0.50 mM; Need ultrasonic); | ||
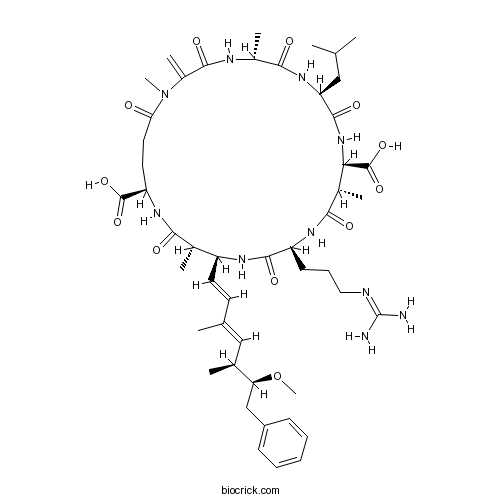
| Chemical Name | (5R,8S,11R,12S,15S,18S,19S,22R)-15-[3-(diaminomethylideneamino)propyl]-18-[(1E,3E,5S,6S)-6-methoxy-3,5-dimethyl-7-phenylhepta-1,3-dienyl]-1,5,12,19-tetramethyl-2-methylidene-8-(2-methylpropyl)-3,6,9,13,16,20,25-heptaoxo-1,4,7,10,14,17,21-heptazacyclopentacosane-11,22-dicarboxylic acid | ||
| SMILES | CC1C(NC(=O)C(NC(=O)C(C(NC(=O)C(NC(=O)C(NC(=O)C(=C)N(C(=O)CCC(NC1=O)C(=O)O)C)C)CC(C)C)C(=O)O)C)CCCN=C(N)N)C=CC(=CC(C)C(CC2=CC=CC=C2)OC)C | ||
| Standard InChIKey | ZYZCGGRZINLQBL-GWRQVWKTSA-N | ||
| Standard InChI | InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Microcystin-LR is a potent inhibitor of protein phosphatase with IC50 of 0.04 nM. | |||||
| Targets | protein phosphatase | |||||
| IC50 | 0.04 nM | |||||

Microcystin-LR Dilution Calculator

Microcystin-LR Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0049 mL | 5.0243 mL | 10.0485 mL | 20.0971 mL | 25.1213 mL |
| 5 mM | 0.201 mL | 1.0049 mL | 2.0097 mL | 4.0194 mL | 5.0243 mL |
| 10 mM | 0.1005 mL | 0.5024 mL | 1.0049 mL | 2.0097 mL | 2.5121 mL |
| 50 mM | 0.0201 mL | 0.1005 mL | 0.201 mL | 0.4019 mL | 0.5024 mL |
| 100 mM | 0.01 mL | 0.0502 mL | 0.1005 mL | 0.201 mL | 0.2512 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 1.7 nM and 0.04 nM for type 1 and type 2A protein phosphatases, respectivley.
Protein phosphatases 1 and 2A, two of the major phosphatases in eukaryotic cells that dephosphorylate serine and threonine residues. Microcystin-LR is a potent inhibitor of type 1 and type 2A protein phosphatases.
In vitro: Microcystin-LR inhibited protein phosphatases 1 (PP1) and 2A (PP2A) with Ki values below 0.1 nM. Protein phosphatase 2B is inhibited 1000-fold less potently, while six other phosphatases and eight protein kinases tested are unaffected. Microcystin-LR inhibited enzymes in a remarkably similar manner to okadaic acid, a potent tumour promoter that is also the toxin responsible for diarrhetic shellfish poisoning [1].
In vivo: After 100 i.p. injections of a sublethal dose (20 μg/kg) of MCLR, neoplastic nodules were observed without the use of an initiator. Multiple neoplastic nodules up to 5 mm in diameter were observed in the liver of mice in both groups, i.e. those injected 100 times i.p. and those injected 100 times with a 2 month withdrawal. In contrast, when 80 μg/kg was orally administered 100 times, characteristic chronic injuries such as fibrous changes and nodule formation were not observed [2].
Clinical trials: Currenlty no clinical data are available.
References:
[1] MacKintosh C, Beattie KA, Klumpp S, Cohen P, Codd GA. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990 May 21;264(2):187-92.
[2] Ito E, Kondo F, Terao K, Harada K. Neoplastic nodular formation in mouse liver induced by repeated intraperitoneal injections of microcystin-LR. Toxicon. 1997 Sep;35(9):1453-7.
- MK-5108 (VX-689)
Catalog No.:BCC2176
CAS No.:1010085-13-8
- Bis[4-(dimethylamino)phenyl]methane
Catalog No.:BCC8889
CAS No.:101-61-1
- Hyoscyamine
Catalog No.:BCN1946
CAS No.:101-31-5
- Pyridostigmine Bromide
Catalog No.:BCC4579
CAS No.:101-26-8
- CY 208-243
Catalog No.:BCC6991
CAS No.:100999-26-6
- Levofloxacin
Catalog No.:BCC4791
CAS No.:100986-85-4
- CX-4945 (Silmitasertib)
Catalog No.:BCC3693
CAS No.:1009820-21-6
- NSC 687852 (b-AP15)
Catalog No.:BCC2389
CAS No.:1009817-63-3
- Thiamet G
Catalog No.:BCC4864
CAS No.:1009816-48-1
- Ebrotidine
Catalog No.:BCC1542
CAS No.:100981-43-9
- Rotundine
Catalog No.:BCN5983
CAS No.:10097-84-4
- 2,2-Bis(hydroxymethyl)butyric acid
Catalog No.:BCC8495
CAS No.:10097-02-6
- Larixinol
Catalog No.:BCN6484
CAS No.:101046-79-1
- Tenovin-3
Catalog No.:BCC3889
CAS No.:1011301-27-1
- 3,8'-Biapigenin
Catalog No.:BCN5825
CAS No.:101140-06-1
- Milnacipran HCl
Catalog No.:BCC4922
CAS No.:101152-94-7
- Odoriflavene
Catalog No.:BCN8240
CAS No.:101153-41-7
- Tenovin-6
Catalog No.:BCC3667
CAS No.:1011557-82-6
- Momordicoside P
Catalog No.:BCN3275
CAS No.:1011726-62-7
- Longipedlactone J
Catalog No.:BCN6644
CAS No.:1011762-93-8
- CUDC-101
Catalog No.:BCC2149
CAS No.:1012054-59-9
- IRAK inhibitor 4
Catalog No.:BCC1657
CAS No.:1012104-68-5
- Picrasidine Q
Catalog No.:BCN3182
CAS No.:101219-61-8
- IRAK inhibitor 3
Catalog No.:BCC1656
CAS No.:1012343-93-9
Quantitative analysis of glutathione and cysteine S-conjugates of microcystin-LR in the liver, kidney and muscle of common carp (Cyprinus carpio) in Lake Taihu.[Pubmed:28362311]
J Water Health. 2017 Apr;15(2):300-307.
Tissue distribution of microcystin (MC)-LR-GSH, MC-LR-Cys and MC-LR of omnivorous fish in Lake Taihu was investigated. MC-LR and MC-LR-Cys were detected in liver, kidney and muscle. The concentration of MC-LR in liver and kidney was 0.052 mug g(-1) DW and 0.067 mug g(-1) DW, respectively. MC-LR-Cys appeared to be an important metabolite with average contents of 1.104 mug g(-1) DW and 0.724 mug g(-1) DW in liver and kidney, and the MC-LR-Cys/MC-LR ratio in liver and kidney reaching as high as 21.4 and 10.8. High MC-LR-Cys/MC-LR ratio and a significant correlation between MC-LR-Cys and MC-LR concentration in liver, suggest that liver is more active in detoxification of MC-LR by formation of MC-LR-Cys for omnivorous fish. Furthermore, there might be a balance between the accumulation and depuration/metabolism of MC-LR-Cys in kidney. The MC-LR-Cys can be formed in kidney directly, or transported from liver or other tissues, while the MC-LR-Cys in kidney might be dissociated to MC-LR or excreted. Although MC-LR and its metabolites were scarcely detected in muscle, it is necessary to investigate the distribution of toxic metabolites in edible muscle.
Microcystin-LR Binds Iron, and Iron Promotes Self-Assembly.[Pubmed:28368104]
Environ Sci Technol. 2017 May 2;51(9):4841-4850.
The microcystin-producing Microcystis aeruginosa PCC 7806 and its close strain, the nonproducing Microcystis aeruginosa PCC 7005, grow similarly in the presence of 17 muM iron. Under severe iron deficient conditions (0.05 muM), the toxigenic strain grows slightly less than in iron-replete conditions, while the nonproducing microcystin strain is not able to grow. Isothermal titration calorimetry performed at cyanobacterial cytosol or meaningful environmental pHs values shows a Microcystin-LR dissociaton constant for Fe(2+) and Fe(3+) of 2.4 muM. Using atomic force microscopy, 40% of Microcystin-LR dimers were observed, and the presence of iron promoted its oligomerization up to six units. Microcystin-LR binds also Mo(6+), Cu(2+), and Mn(2+). Polymeric microcystin binding iron may be related with a toxic cell colony advantage, providing enhanced iron bioavailability and perhaps affecting the structure of the gelatinous sheath. Inside cells, with microcystin implicated in the fitness of the photosynthetic machinery under stress conditions, the toxin would be involved in avoiding metal-dependent Fenton reactions when photooxidation causes disassembly of the iron-rich photosystems. Additionally, it could be hypothesized that polymerization-depolymerization dynamics may be an additional signal that could trigger changes (for example, in the binding of microcystin to proteins).
Altered cellular metabolism of HepG2 cells caused by microcystin-LR.[Pubmed:28336091]
Environ Pollut. 2017 Jun;225:610-619.
This study aimed to evaluate the possible effects of Microcystin-LR (MC-LR) exposure on the metabolism and drug resistance of human hepatocellular carcinoma (HepG2) cells. For this purpose, we first conducted an experiment to make sure that MC-LR could penetrate the HepG2 cell membrane effectively. The transcriptional levels of phase I (such as CYP2E1, CYP3A4, and CYP26B1) and phase II (such as EPHX1, SULTs, and GSTM) enzymes and export pump genes (such as MRP1 and MDR1) were altered by MC-LR-exposure for 24 h, indicating that MC-LR treatment may destabilize the metabolism of HepG2 cells. Further research showed that the CYP inducers omeprazole, ethanol, and rifampicin inhibited cell viability, in particular, ethanol, a CYP2E1 inducer, induced ROS generation, lipid peroxidation, and apoptosis in HepG2 cells treated with MC-LR. The CYP2E1 inhibitor chlormethiazole inhibited ROS generation, mitochondrial membrane potential loss, caspase-3 activity, and cytotoxicity caused by MC-LR. Meanwhile, the results also showed that co-incubation with the ROS scavenger l-ascorbic acid and MC-LR decreased ROS levels and effectively prevented apoptosis. These findings provide an interesting mechanistic explanation of cellular metabolism associated with MC-LR, i.e., MC-LR-exposure exerted toxicity on HepG2 cells and induced apoptosis of HepG2 cells via promoting CYP2E1 expression and inducing excessive ROS in HepG2 cells.
Photorelease of microcystin-LR from resuspended sediments.[Pubmed:28366384]
Harmful Algae. 2017 Mar;63:1-6.
A series of ten photolysis experiments was conducted with sediments exposed to Microcystis sp. blooms to determine if sunlight is capable of mobilizing the biotoxin Microcystin-LR (MC-LR) into the water column. There was a net photorelease of MC-LR in irradiated suspensions in all cases relative to dark controls, ranging from 0.4 to 192mugL(-1)g(-1) into the dissolved phase. This should be viewed as a minimum estimate of photorelease due to concurrent photodegradation of dissolved toxin. Dissolved MC-LR concentrations in a sediment suspension increased linearly in the aqueous phase during a six-hour irradiation with simulated sunlight suggesting that longer exposure times produce greater quantities of MC-LR. There was a significant positive correlation between photorelease of toxin and percent organic carbon of the resuspended material, implying that organic-rich sediments yield the greatest photorelease of MC-LR upon exposure to full spectrum sunlight. Samples exposed to photosynthetically active radiation (400nm-700nm) were responsible for less than 2% of the photorelease compared to full spectrum exposures. Model calculations indicate that photochemical processing of bloom impacted sediments could be responsible for as much as 100% of the average standing stock of MC-LR in a freshwater pond located in southeastern North Carolina, where surface water concentrations were also measured. Mass spectrometric analysis revealed a new peak in light exposed flasks that appears to be a photo-induced isomerized product of MC-LR. Photoproduction from resuspended sediments therefore represents a significant but previously unrecognized source of highly toxic MC-LR and photoproducts of unknown toxicity and fate to aquatic ecosystems.


