LevofloxacinCAS# 100986-85-4 |
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 100986-85-4 | SDF | Download SDF |
| PubChem ID | 3033924 | Appearance | Powder |
| Formula | C18H20FN3O4 | M.Wt | 361.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (-)-Ofloxacin | ||
| Solubility | H2O : 50 mg/mL (138.36 mM; Need ultrasonic) DMSO : 10 mg/mL (27.67 mM; Need ultrasonic) | ||
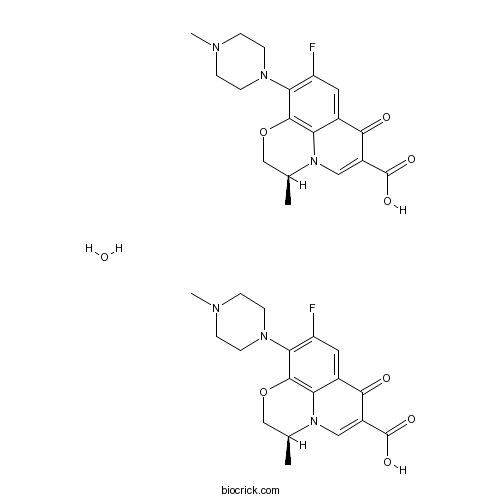
| SMILES | CC1COC2=C3N1C=C(C(=O)C3=CC(=C2N4CCN(CC4)C)F)C(=O)O.CC1COC2=C3N1C=C(C(=O)C3=CC(=C2N4CCN(CC4)C)F)C(=O)O.O | ||
| Standard InChIKey | SUIQUYDRLGGZOL-RCWTXCDDSA-N | ||
| Standard InChI | InChI=1S/2C18H20FN3O4.H2O/c2*1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21;/h2*7-8,10H,3-6,9H2,1-2H3,(H,24,25);1H2/t2*10-;/m00./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Levofloxacin, a synthetic fluoroquinolone, is an antibacterial agent that inhibits the supercoiling activity of bacterial DNA gyrase, halting DNA replication.
Target: Antibacterial
Levofloxacin reduced bacterial load compared with placebo by 4.9-fold (95% confidence interval, 1.4-25.7; P=0.02) at day 7 but had no effect at any point on any marker of neutrophilic airway inflammation. In patients with a baseline bacterial load of more than 10(6) cfu/mL, levofloxacin treatment was associated with a 26.5% (95% confidence interval, 1.8%-51.3%; P=0.04) greater reduction in the percentage neutrophil count compared with placebo at day 7 [1]. Levofloxacin was found to significantly improve the clinical and microbiological parameters in CP individuals [2]. A 30-day course of levofloxacin does not significantly improve BK viral load reduction or allograft function when used in addition to overall reduction of immunosuppression [3]. References: | |||||

Levofloxacin Dilution Calculator

Levofloxacin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7672 mL | 13.8362 mL | 27.6725 mL | 55.3449 mL | 69.1812 mL |
| 5 mM | 0.5534 mL | 2.7672 mL | 5.5345 mL | 11.069 mL | 13.8362 mL |
| 10 mM | 0.2767 mL | 1.3836 mL | 2.7672 mL | 5.5345 mL | 6.9181 mL |
| 50 mM | 0.0553 mL | 0.2767 mL | 0.5534 mL | 1.1069 mL | 1.3836 mL |
| 100 mM | 0.0277 mL | 0.1384 mL | 0.2767 mL | 0.5534 mL | 0.6918 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Levofloxacin, a synthetic fluoroquinolone, is an antibacterial agent that inhibits the supercoiling activity of bacterial DNA gyrase, halting DNA replication.
- CX-4945 (Silmitasertib)
Catalog No.:BCC3693
CAS No.:1009820-21-6
- NSC 687852 (b-AP15)
Catalog No.:BCC2389
CAS No.:1009817-63-3
- Thiamet G
Catalog No.:BCC4864
CAS No.:1009816-48-1
- Ebrotidine
Catalog No.:BCC1542
CAS No.:100981-43-9
- Rotundine
Catalog No.:BCN5983
CAS No.:10097-84-4
- 2,2-Bis(hydroxymethyl)butyric acid
Catalog No.:BCC8495
CAS No.:10097-02-6
- Stachyose
Catalog No.:BCN2566
CAS No.:10094-58-3
- Caulophyllumine A
Catalog No.:BCN7928
CAS No.:1009318-60-8
- AZD2014
Catalog No.:BCC3732
CAS No.:1009298-59-2
- AZD8055
Catalog No.:BCC3629
CAS No.:1009298-09-2
- Panamycin 607
Catalog No.:BCN1813
CAS No.:100905-89-3
- Piceatannol
Catalog No.:BCN5824
CAS No.:10083-24-6
- CY 208-243
Catalog No.:BCC6991
CAS No.:100999-26-6
- Pyridostigmine Bromide
Catalog No.:BCC4579
CAS No.:101-26-8
- Hyoscyamine
Catalog No.:BCN1946
CAS No.:101-31-5
- Bis[4-(dimethylamino)phenyl]methane
Catalog No.:BCC8889
CAS No.:101-61-1
- MK-5108 (VX-689)
Catalog No.:BCC2176
CAS No.:1010085-13-8
- Microcystin-LR
Catalog No.:BCC5339
CAS No.:101043-37-2
- Larixinol
Catalog No.:BCN6484
CAS No.:101046-79-1
- Tenovin-3
Catalog No.:BCC3889
CAS No.:1011301-27-1
- 3,8'-Biapigenin
Catalog No.:BCN5825
CAS No.:101140-06-1
- Milnacipran HCl
Catalog No.:BCC4922
CAS No.:101152-94-7
- Odoriflavene
Catalog No.:BCN8240
CAS No.:101153-41-7
- Tenovin-6
Catalog No.:BCC3667
CAS No.:1011557-82-6
Meropenem, levofloxacin and linezolid in human plasma of critical care patients: A fast semi-automated micro-extraction by packed sorbent UHPLC-PDA method for their simultaneous determination.[Pubmed:28371721]
J Pharm Biomed Anal. 2017 Jun 5;140:266-273.
An ultra high-performance liquid chromatographic (UHPLC) method with PDA detection was developed and validated for the simultaneous quantification of meropenem, linezolid, and Levofloxacin in human plasma and applied in human plasma of critical care patients. A semi-automated microextraction by packed sorbent (MEPS) for sample preparation was used. All parameters in the extraction step (pH, sample volume, sample dilution and number of aspiration - ejection cycles) and in the desorption step (percentage of acetonitrile in the solvent of elution and number of aspirations of elution solvent through the device) were statistically significant when the recovery was used as response. The method showed good linearity with correlation coefficients, r(2)>0.9991 for the three drugs, as well as high precision (RSD%<10.83% in each case). Accuracy ranged from -7.8% to +6.7%. The limit of quantification of the three drugs was established at 0.01mug/mL for linezolid and Levofloxacin and 0.02mug/mL for meropenem. Linezolid, meropenem, Levofloxacin and the internal standard were extracted from human plasma with a mean recovery ranged from 92.4% to 97.4%. During validation, the concentration of meropenem, linezolid and Levofloxacin was found to be stable after 3 freeze-thaw cycles and for at least 24h after extraction. This method will be subsequently used to quantify the drugs in patients to establish if the dosage regimen given is sufficient to eradicate the infection at the target site.
Gold Nanoparticles Conjugated Levofloxacin: For Improved Antibacterial Activity Over Levofloxacin Alone.[Pubmed:28302030]
Curr Drug Deliv. 2017;14(8):1114-1119.
BACKGROUND: Levofloxacin is a potent antibiotic with severe side effects due to its high doses. Bacterial resistance may be due to frequent use of antibiotics. Biogenic gold nanoparticles conjugated Levofloxacin (Au-HSA-LvN-NPs) were developed by Human Serum Albumin (HSA) and nitrate reductasemediated pathways. METHODS: Au-HSA-LvN-NPs (size = 27.2 +/- 1 nm) were readily generated with high emulsion stability zeta potential (-13.3 mV). The developed nanoparticles were also characterized by UVvisible spectroscopy, Transmission Electron Microscopy and Dynamic Light Scattering techniques. RESULTS: The optimized nanoparticles were found efficient against both Gram-positive bacteria and Gramnegative bacteria specifically S. aureus (MIC-0.373 microg/ml), E. coli (MIC-0.149 microg/ml) and P. aeruginosa (MIC-0.346 microg/ml) respectively. CONCLUSION: The efficiency of bioconjugated Levofloxacin got improved by 1.94 times, 2.89 times and 1.46 times against S. aureus, E. coli and P. aeruginosa respectively, in comparison to pure Levofloxacin.
Effect of the administration period of perioperative topical levofloxacin on normal conjunctival bacterial flora.[Pubmed:28317676]
J Cataract Refract Surg. 2017 Jan;43(1):42-48.
PURPOSE: To assess the long-term and short-term effects of post-cataract surgery antibiotic therapy on the drug-resistance profile of normal conjunctival bacterial flora. SETTING: Miyata Eye Hospital, Miyazaki, Japan. DESIGN: Randomized prospective clinical trial. METHODS: Patients aged 20 years or older who had cataract surgery between May and September 2015 were given Levofloxacin 1.5% ophthalmic solution for 3 days preoperatively. The patients were randomly assigned to a 1-week postoperative group or a 1-month postoperative group according to postoperative administration duration. Conjunctival sacs were scraped for bacterial culturing before administration, 1 week postoperatively, at the completion of administration, and 1, 3, and 6 months after administration completion. The bacterial culture growth and minimum inhibitory concentrations (MICs) of Levofloxacin against recovered strains of Staphylococcus epidermidis were assessed. RESULTS: The study enrolled 104 patients. The MICs of Levofloxacin against S epidermidis increased during Levofloxacin administration compared with before administration in both groups and then declined after administration completion. However, by 3 months, the MICs in the 1-month group were approximately twice those in the 1-week group. Antibiotic susceptibility before administration, at completion of administration, and at 3 months was 73.6%, 20.2%, and 38.5%, respectively, in the 1-week group and 63.0%, 0.0%, and 19.3%, respectively, in the 1-month group. The results indicate that from completion of administration to 3 months, the susceptible strains were approximately 20% lower in the 1-month postoperative group than in the 1-week postoperative group. CONCLUSION: Administration duration of perioperative Levofloxacin 1.5% influenced the MICs and susceptibility of S epidermidis isolated from the conjunctival sac.
A prospective evaluation of levofloxacin-based triple therapy for refractory Helicobacter pylori infection in Australia.[Pubmed:28345276]
Intern Med J. 2017 Jul;47(7):761-766.
BACKGROUND: First-line Helicobacter pylori eradication failure is a common and challenging problem. AIM: To assess the efficacy of salvage Levofloxacin-based triple therapy in Australia. METHODS: Prospective patients referred after prior treatment failure(s) were prescribed esomeprazole 40 mg, amoxicillin 1 g and Levofloxacin 500 mg each twice daily for 10 days. All patients received detailed written and verbal adherence support. Outcome assessment was by (13) C-urea breath test and/or histology and urease test. RESULTS: In 150 consecutive, evaluable patients (66% female, mean age 54 +/- 14 years; six smokers), the main indications for treatment were peptic ulcer disease (17%), increased gastric cancer risk (20%), symptoms (35%) and other risk reduction (28%). The median number of previous treatments was 2 (range 1-7). Eradication of H. pylori was achieved in 90% (intention to treat (ITT)) and 91% (per-protocol (PP)) of patients. The eradication rate did not differ according to the type or number of prior treatments: 93% when Levofloxacin resistance was observed in 20 concurrent cases. CONCLUSION: The efficacy and safety of this Levofloxacin-based triple therapy suggests it should be used as a salvage regimen in this region. Randomised comparative trials are unlikely to be done but these data compare favourably with local data for other salvage therapies.


