RotundineDopamine D1 receptor antagonist,potent and selective CAS# 10097-84-4 |
- Perindopril Erbumine
Catalog No.:BCC3586
CAS No.:107133-36-8
- Losartan Potassium (DuP 753)
Catalog No.:BCC1080
CAS No.:124750-99-8
- Candesartan
Catalog No.:BCC2558
CAS No.:139481-59-7
- Telmisattan
Catalog No.:BCC3863
CAS No.:144701-48-4
- Imidapril HCl
Catalog No.:BCC3792
CAS No.:89396-94-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 10097-84-4 | SDF | Download SDF |
| PubChem ID | 72301 | Appearance | White powder |
| Formula | C21H25NO4 | M.Wt | 355.42 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | (-)-Tetrahydropalmatine;483-14-7 | ||
| Solubility | Soluble in DMSO > 10 mM | ||
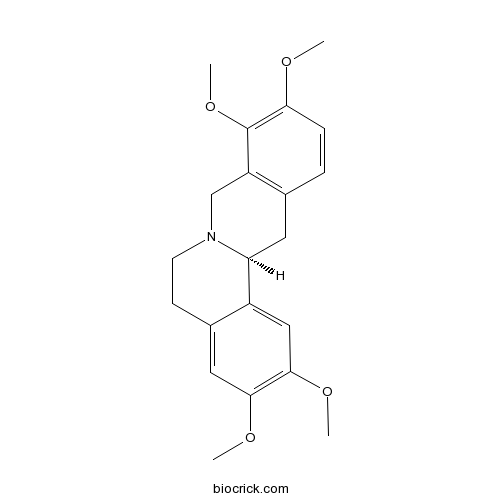
| Chemical Name | (13aS)-2,3,9,10-tetramethoxy-6,8,13,13a-tetrahydro-5H-isoquinolino[2,1-b]isoquinoline | ||
| SMILES | COC1=C(C2=C(CC3C4=CC(=C(C=C4CCN3C2)OC)OC)C=C1)OC | ||
| Standard InChIKey | AEQDJSLRWYMAQI-KRWDZBQOSA-N | ||
| Standard InChI | InChI=1S/C21H25NO4/c1-23-18-6-5-13-9-17-15-11-20(25-3)19(24-2)10-14(15)7-8-22(17)12-16(13)21(18)26-4/h5-6,10-11,17H,7-9,12H2,1-4H3/t17-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Rotundine provides remarkable protection against cerebral ischemia reperfusion damage, its mechanisms may be through its influence on NO、ET 1 and energy metabolism. Rotundine injection can ameliorate the damages by modulating the activities of different types of NOS. It combined with methadone treatment may be a better therapeutic method in treatment of heroin dependence.Rotundine (L-tetrahydropalmatine, L-THP) is a selective antagonist of dopamine D1 receptor with IC50 of 166 nM. |
| Targets | NOS | NO | D1 receptor | 5-HT1A | D2 receptor | D3 receptor |
| In vivo | Therapeutic Effects of Rotundine Combined with Methadone in Treatment of Heroin Dependence[Reference: WebLink]Chinese Journal of Drug Abuse Prevention & Treatment, 2006, 12(5):270-1.To explore the therapeutic effects of Rotundine combined with methadone in treatment of heroin dependence.
Influence of rotundine on NO、ET-1 and energy metabolism after acute brain injury resulted from complete cerebral ischemia reperfusion in the rats[Reference: WebLink]Journal of Emergenoy Medicine, 2000, 9(6):379-81.
|
| Animal Research | Effects of rotundine injection on nitric oxide synthase in tissues of different organs after ischemia/reperfusion of brain in rats.[Pubmed: 17577446]Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2007 Jun;19(6):361-4.To explore the protective effect of Rotundine injection on lung, liver and kidney damages after cerebral ischemia/reperfusion (I/R) injury in rats based on the activity changes of nitric oxide synthase (NOS).
|
| Structure Identification | Guang Pu Xue Yu Guang Pu Fen Xi. 2014 Nov;34(11):2989-93.IR and Raman spectra studies of Rotundine based on DFT.[Pubmed: 25752044]Infrared spectroscopy (IR), the normal Raman spectroscopy (NRS) and the surface enhanced Raman spectroscopy (SERS) in new Ag/Cu nanomaterial of Rotundine were studied in the present paper.
|

Rotundine Dilution Calculator

Rotundine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8136 mL | 14.0679 mL | 28.1357 mL | 56.2715 mL | 70.3393 mL |
| 5 mM | 0.5627 mL | 2.8136 mL | 5.6271 mL | 11.2543 mL | 14.0679 mL |
| 10 mM | 0.2814 mL | 1.4068 mL | 2.8136 mL | 5.6271 mL | 7.0339 mL |
| 50 mM | 0.0563 mL | 0.2814 mL | 0.5627 mL | 1.1254 mL | 1.4068 mL |
| 100 mM | 0.0281 mL | 0.1407 mL | 0.2814 mL | 0.5627 mL | 0.7034 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Levo-tetrahydropalmatine (l-THP) is an active herbal constituent of preparations containing plant species of the genera Stephania and Corydalis. In China, it has been approved and used for a number of clinical indications under the drug name Rotundine.
In vitro: In contrast to other THPB derivatives, l-THP is an antagonist at both of D1 and D2 receptors. The Ki values for l-THP at D1 and D2 dopamine receptors are approximately 124 nM (D1) and 388 nM (D2), while the IC50 values are 166 nM and 1.4 μM for D1 and D2, respectively. The relatively high affinity of l-THP at D1 vs. D2 receptors, distinguishes it from other available dopamine receptor antagonist drugs (e.g., haloperidol) [1].
In vivo: It has been found that l-THP produces dose-dependent reductions in locomotor activity and operant responding for non-drug reinforcers in rats [1].
Clinical trials: It has long been recognized l-THP has therapeutic values for treating a number of CNS related conditions. The effectiveness of l-THP as a non-opioid analgesic agent resulted in the approval of purified l-THP for this indication by the Chinese SFDA and has led to extensive investigation of the pharmacological properties of l-THP [1]
Reference:
[1] Wang JB, Mantsch JR. l-tetrahydropalamatine: a potential new medication for the treatment of cocaine addiction. Future Med Chem. 2012 Feb;4(2):177-86.
- 2,2-Bis(hydroxymethyl)butyric acid
Catalog No.:BCC8495
CAS No.:10097-02-6
- Stachyose
Catalog No.:BCN2566
CAS No.:10094-58-3
- Caulophyllumine A
Catalog No.:BCN7928
CAS No.:1009318-60-8
- AZD2014
Catalog No.:BCC3732
CAS No.:1009298-59-2
- AZD8055
Catalog No.:BCC3629
CAS No.:1009298-09-2
- Panamycin 607
Catalog No.:BCN1813
CAS No.:100905-89-3
- Piceatannol
Catalog No.:BCN5824
CAS No.:10083-24-6
- (RS)-CPP
Catalog No.:BCC6561
CAS No.:100828-16-8
- 4,4'-Bis(α,α-dimethylbenzyl)diphenylamine
Catalog No.:BCC8661
CAS No.:10081-67-1
- Chlorahololide C
Catalog No.:BCN7256
CAS No.:1007859-25-7
- (R)-5-Hydroxy-1,7-diphenyl-3-heptanone
Catalog No.:BCN3591
CAS No.:100761-20-4
- Ganoderic acid M
Catalog No.:BCN2871
CAS No.:100761-17-9
- Ebrotidine
Catalog No.:BCC1542
CAS No.:100981-43-9
- Thiamet G
Catalog No.:BCC4864
CAS No.:1009816-48-1
- NSC 687852 (b-AP15)
Catalog No.:BCC2389
CAS No.:1009817-63-3
- CX-4945 (Silmitasertib)
Catalog No.:BCC3693
CAS No.:1009820-21-6
- Levofloxacin
Catalog No.:BCC4791
CAS No.:100986-85-4
- CY 208-243
Catalog No.:BCC6991
CAS No.:100999-26-6
- Pyridostigmine Bromide
Catalog No.:BCC4579
CAS No.:101-26-8
- Hyoscyamine
Catalog No.:BCN1946
CAS No.:101-31-5
- Bis[4-(dimethylamino)phenyl]methane
Catalog No.:BCC8889
CAS No.:101-61-1
- MK-5108 (VX-689)
Catalog No.:BCC2176
CAS No.:1010085-13-8
- Microcystin-LR
Catalog No.:BCC5339
CAS No.:101043-37-2
- Larixinol
Catalog No.:BCN6484
CAS No.:101046-79-1
[Enhancing effect of volatile oils of rhizoma zingiberis, flos magnoliae and fructus litseae on permeation of rotundine in vitro].[Pubmed:21954565]
Zhong Yao Cai. 2011 May;34(5):753-7.
OBJECTIVE: To investigate the enhancing effect of volatile oils of Rhizoma Zingiberis (RZ), Flos Magnoliae (FM), Fructus Litseae (FL), Azone and pairwise combinations on the permeation of Rotundine in vitro. METHODS: To screen out the volatile oils with the best percutaneous enhancing effect on Rotundine, a test of penetration through rats skin was conducted by using an improved Franz diffusion cell. The accumulative penetration amount of Rotundine was determined by UV and HPLC. RESULTS: All of the 3 volatile oils had enhancing effect on Rotundine permeation. The average accumulative doses of Rotundine for 8 hours (Q8, mg/cm2) of 5% volatile oil of FL, 5% volatile oil of, RZ, 2.5% FL + 2.5% Azone were 6.0758, 6.1148, 6.5487, the enhancement ratios of 4 hours were 1.01, 1.00, 1.13 respectively. CONCLUSION: 2.5% FL + 2.5% Azone had the best effect of percutaneous enhancing on Rotundine.
[Effects of rotundine injection on nitric oxide synthase in tissues of different organs after ischemia/reperfusion of brain in rats].[Pubmed:17577446]
Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2007 Jun;19(6):361-4.
OBJECTIVE: To explore the protective effect of Rotundine injection on lung, liver and kidney damages after cerebral ischemia/reperfusion (I/R) injury in rats based on the activity changes of nitric oxide synthase (NOS). METHODS: Seventy-six rats were randomly divided into three groups: sham operation group, I/R injury group and treatment group, and determinations were done at five different time points. The cerebral I/R models were reproduced by improved 4 vessels occlusion method. The activities of NOS in the lung, liver and kidney were measured in all the rats at 2, 6, 12, 24 and 48 hours after reperfusion. RESULTS: Compared with sham operation group, the activities of total NOS (tNOS) were significantly increased at 2, 12 and 24 hours in I/R injury group (P<0.05 or P<0.01), with the peak value at 12 hours (all P<0.01). The activities of constitutive NOS (cNOS) were increased significantly at 2 hours (all P<0.05), and those of induced NOS (iNOS) were increased at 12 hours (all P<0.01). The activities of iNOS were still high at 24 hours (all P<0.05), and approached the levels of sham operation group at 48 hours. Compared with I/R injury group, the activities of cNOS in various organs increased much higher at 2 hours in treatment group (all P<0.05). But those of iNOS were significantly decreased after 12 hours (P<0.05 or P<0.01). CONCLUSION: The various types of NOS play different roles in the lung damages after brain I/R injury at different stages in rats. Rotundine injection can ameliorate the damages by modulating the activities of different types of NOS.
[IR and Raman spectra studies of Rotundine based on DFT].[Pubmed:25752044]
Guang Pu Xue Yu Guang Pu Fen Xi. 2014 Nov;34(11):2989-93.
Infrared spectroscopy (IR), the normal Raman spectroscopy (NRS) and the surface enhanced Raman spectroscopy (SERS) in new Ag/Cu nanomaterial of Rotundine were studied in the present paper. The IR and the NRS of Rotundine were calculated by the density functional theory (DFT) using B3LYP/6-311+G(d, p), then the spectral intensity graph of Rotundine were given. The vibrational peaks were assigned comprehensively by the visualization software of Gauss view 5. 0. Rotundine has obvious infrared and Raman vibrational peak in the wave number range of 3 300-2500 and 1 800-600 cm(-1). SnCl2 and PVP was used as capping agent for the silver nanoparticles in SERS of Rotundine. Finally, by using the method of cyclic immersion well dispersed silver nanoparticles was obtained and achieved good enhancement effect. This molecule acquired strong selective enhancement vibration peak, In the wave number ranges of 1 500-1 400 and 1 000-700 cm(-1) the enhancement effect is most obvious. After analyzed, the methylene of this molecule is adsorbed on the silver nanoparticles surface and the angle between the benzene ring and the silver substrate is close to 90 degrees. The theoretically calculated spectra of Rotundine are consistent with the obtained experimental spectra. There are some differences may be due to the interaction forces between molecules and so on. The visualization software displayed the structure characteristics and molecular group vibration of this molecular visually and provided important basis for assigning the vibrational peaks. Rotundine is an important traditional Chinese medicine agent contained in many kinds of sedative drugs. The study provides a strong basis for the rapid, feature and trace identification of Rotundine and also supplies important reference for the biological role of central inhibition of analgesic drugs.
[In vitro O-demethylation of rotundine by recombinant human CYP isoenzymes].[Pubmed:21351505]
Yao Xue Xue Bao. 2010 Mar;45(3):307-13.
Rotundine (1 micromol L(-1)) was incubated with a panel of rCYP enzymes (1A2, 2C9, 2C19, 2D6 and 3A4) in vitro. The remained parent drug in incubates was quantitatively analyzed by an Agilent LC-MS. CYP2C19, 3A4 and 2D6 were identified to be the isoenzymes involved in the metabolism of Rotundine. The individual contributions of CYP2C19, 3A4 and 2D6 to the Rotundine metabolism were assessed using the method of total normalized rate to be 31.46%, 60.37% and 8.17%, respectively. The metabolites of Rotundine in incubates were screened with ESI-MS at selected ion mode, and were further identified using MS2 spectra and precise molecular mass obtained from an Agilent LC/Q-TOF-MSMS, as well as MS(n) spectra of LC-iTrap-MS(n). The predominant metabolic pathway of Rotundine in rCYP incubates was O-demethylation. A total 5 metabolites were identified including 4 isomerides of mono demethylated Rotundine and one di-demethylated metabolite. The results also showed that CYP2C19, 2D6 and 3A4 mediated O-demethylation of methoxyl groups at different positions of Rotundine. Furthermore, the ESI-MS cleavage patterns of Rotundine and its metabolites were explored by using LC/Q-TOF-MSMS and LC/iTrap-MS(n) techniques.


