Thiamet GO-GlcNAcase inhibitor, potent and selective CAS# 1009816-48-1 |
- Lenalidomide hydrochloride
Catalog No.:BCC1697
CAS No.:1243329-97-6
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- Necrostatin 2 racemate
Catalog No.:BCC2077
CAS No.:852391-15-2
- Necrostatin 2
Catalog No.:BCC1793
CAS No.:852391-19-6
- Necrostatin 2 S enantiomer
Catalog No.:BCC2078
CAS No.:852391-20-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1009816-48-1 | SDF | Download SDF |
| PubChem ID | 24808478 | Appearance | Powder |
| Formula | C9H16N2O4S | M.Wt | 248.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 50 mg/mL (201.37 mM) DMSO : ≥ 45 mg/mL (181.23 mM) *"≥" means soluble, but saturation unknown. | ||
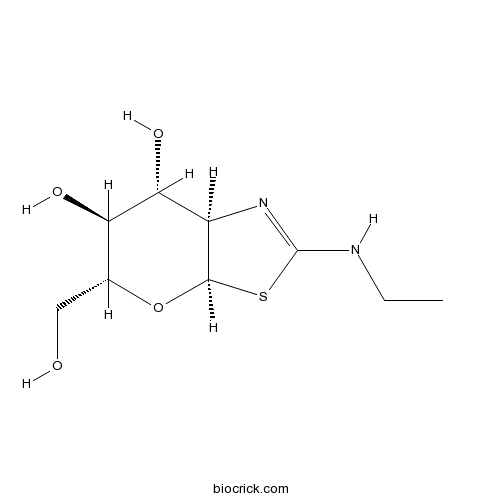
| Chemical Name | (3aR,5R,6S,7R,7aR)-2-(ethylamino)-5-(hydroxymethyl)-5,6,7,7a-tetrahydro-3aH-pyrano[3,2-d][1,3]thiazole-6,7-diol | ||
| SMILES | CCNC1=NC2C(C(C(OC2S1)CO)O)O | ||
| Standard InChIKey | PPAIMZHKIXDJRN-FMDGEEDCSA-N | ||
| Standard InChI | InChI=1S/C9H16N2O4S/c1-2-10-9-11-5-7(14)6(13)4(3-12)15-8(5)16-9/h4-8,12-14H,2-3H2,1H3,(H,10,11)/t4-,5-,6-,7-,8-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, selective inhibitor of O-GlcNAcase (Ki = 21 nM for human O-GlcNAcase). Decreases the phosphorylation of tau protein in vivo. Promotes autophagy independently of mTOR pathway and reduces toxic protein species in mouse tauopathy model. Orally bioavailable and blood brain barrier permeable. |

Thiamet G Dilution Calculator

Thiamet G Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0274 mL | 20.1369 mL | 40.2739 mL | 80.5477 mL | 100.6847 mL |
| 5 mM | 0.8055 mL | 4.0274 mL | 8.0548 mL | 16.1095 mL | 20.1369 mL |
| 10 mM | 0.4027 mL | 2.0137 mL | 4.0274 mL | 8.0548 mL | 10.0685 mL |
| 50 mM | 0.0805 mL | 0.4027 mL | 0.8055 mL | 1.611 mL | 2.0137 mL |
| 100 mM | 0.0403 mL | 0.2014 mL | 0.4027 mL | 0.8055 mL | 1.0068 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Thiamet G is a potent and selective inhibitor of O-GlcNAcase with Ki value of 21 nM [1].
O-GlcNAcase is an enzyme that removes GlcNAc from proteins. O-GlcNAcylation refers to the posttranslational modification of O-linkage of N-acetyl-glucosamine moieties to threonine and serine residues on proteins [2].
Thiamet G is a potent and selective O-GlcNAcase inhibitor. In kinetic assays, thiamet G competitively inhibited human O-GlcNAcase with Ki value of 21 nM. Thiamet G was extremely stable in aqueous solution. In nerve growth factor (NGF)-differentiated PC-12 cells, thiamet G significantly increased cellular O-GlcNAc levels with EC50 value of 30 nM in a dose dependent way. Thiamet G significantly reduced phosphorylation levels at Ser396 and Thr231 of Tau by 2.1-fold and 2.7-fold, respectively. Also, thiamet G decreased the phosphorylation levels at Ser422 and Ser262 [1]. Thiamet G significantly sensitized human leukemia cell lines to paclitaxel, a microtubule-stabilizing agent [2].
In rats, thiamet G could readily cross the blood brain barrier and dose-dependently increased brain O-GlcNAc levels. Thiamet G reduced tau phosphorylation at Ser396, Thr231 and Ser422 by 2.7, 3.1 and 1.8-fold, respectively [1].
References:
[1]. Yuzwa SA, Macauley MS, Heinonen JE, et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol, 2008, 4(8): 483-490.
[2]. Ding N, Ping L, Shi Y, et al. Thiamet-G-mediated inhibition of O-GlcNAcase sensitizes human leukemia cells to microtubule-stabilizing agent paclitaxel. Biochem Biophys Res Commun, 2014, 453(3): 392-397.
- Ebrotidine
Catalog No.:BCC1542
CAS No.:100981-43-9
- Rotundine
Catalog No.:BCN5983
CAS No.:10097-84-4
- 2,2-Bis(hydroxymethyl)butyric acid
Catalog No.:BCC8495
CAS No.:10097-02-6
- Stachyose
Catalog No.:BCN2566
CAS No.:10094-58-3
- Caulophyllumine A
Catalog No.:BCN7928
CAS No.:1009318-60-8
- AZD2014
Catalog No.:BCC3732
CAS No.:1009298-59-2
- AZD8055
Catalog No.:BCC3629
CAS No.:1009298-09-2
- Panamycin 607
Catalog No.:BCN1813
CAS No.:100905-89-3
- Piceatannol
Catalog No.:BCN5824
CAS No.:10083-24-6
- (RS)-CPP
Catalog No.:BCC6561
CAS No.:100828-16-8
- 4,4'-Bis(α,α-dimethylbenzyl)diphenylamine
Catalog No.:BCC8661
CAS No.:10081-67-1
- Chlorahololide C
Catalog No.:BCN7256
CAS No.:1007859-25-7
- NSC 687852 (b-AP15)
Catalog No.:BCC2389
CAS No.:1009817-63-3
- CX-4945 (Silmitasertib)
Catalog No.:BCC3693
CAS No.:1009820-21-6
- Levofloxacin
Catalog No.:BCC4791
CAS No.:100986-85-4
- CY 208-243
Catalog No.:BCC6991
CAS No.:100999-26-6
- Pyridostigmine Bromide
Catalog No.:BCC4579
CAS No.:101-26-8
- Hyoscyamine
Catalog No.:BCN1946
CAS No.:101-31-5
- Bis[4-(dimethylamino)phenyl]methane
Catalog No.:BCC8889
CAS No.:101-61-1
- MK-5108 (VX-689)
Catalog No.:BCC2176
CAS No.:1010085-13-8
- Microcystin-LR
Catalog No.:BCC5339
CAS No.:101043-37-2
- Larixinol
Catalog No.:BCN6484
CAS No.:101046-79-1
- Tenovin-3
Catalog No.:BCC3889
CAS No.:1011301-27-1
- 3,8'-Biapigenin
Catalog No.:BCN5825
CAS No.:101140-06-1
Thiamet G mediates neuroprotection in experimental stroke by modulating microglia/macrophage polarization and inhibiting NF-kappaB p65 signaling.[Pubmed:27864466]
J Cereb Blood Flow Metab. 2017 Aug;37(8):2938-2951.
Inflammatory responses are accountable for secondary injury induced by acute ischemic stroke (AIS). Previous studies indicated that O-GlcNAc modification (O-GlcNAcylation) is involved in the pathology of AIS, and increase of O-GlcNAcylation by glucosamine attenuated the brain damage after ischemia/reperfusion. Inhibition of beta-N-acetylglucosaminidase (OGA) with Thiamet G (TMG) is an alternative option for accumulating O-GlcNAcylated proteins. In this study, we investigate the neuroprotective effect of TMG in a mouse model of experimental stroke. Our results indicate that TMG administration either before or after middle cerebral artery occlusion (MCAO) surgery dramatically reduced infarct volume compared with that in untreated controls. TMG treatment ameliorated the neurological deficits and improved clinical outcomes in neurobehavioral tests by modulating the expression of pro-inflammatory and anti-inflammatory cytokines. Additionally, TMG administration reduced the number of Iba1(+) cells in MCAO mice, decreased expression of the M1 markers, and increased expression of the M2 markers in vivo. In vitro, M1 polarization of BV2 cells was inhibited by TMG treatment. Moreover, TMG decreased the expression of iNOS and COX2 mainly by suppressing NF-kappaB p65 signaling. These results suggest that TMG exerts a neuroprotective effect and could be useful as an anti-inflammatory agent for ischemic stroke therapy.
Thiamet-G-mediated inhibition of O-GlcNAcase sensitizes human leukemia cells to microtubule-stabilizing agent paclitaxel.[Pubmed:25268318]
Biochem Biophys Res Commun. 2014 Oct 24;453(3):392-7.
Although the microtubule-stabilizing agent paclitaxel has been widely used for treatment of several cancer types, particularly for the malignancies of epithelia origin, it only shows limited efficacy on hematological malignancies. Emerging roles of O-GlcNAcylation modification of proteins in various cancer types have implicated the key enzymes catalyzing this reversible modification as targets for cancer therapy. Here, we show that the highly selective O-GlcNAcase (OGA) inhibitor thiamet-G significantly sensitized human leukemia cell lines to paclitaxel, with an approximate 10-fold leftward shift of IC50. Knockdown of OGA by siRNAs or inhibition of OGA by thiamet-G did not influence the cell viability. Furthermore, we demonstrated that thiamet-G binds to OGA in competition with 4-methylumbelliferyl N-acetyl-beta-d-glucosaminide dehydrate, an analogue of O-GlcNAc UDP, thereby suppressing the activity of OGA. Importantly, inhibition of OGA by thiamet-G decreased the phosphorylation of microtubule-associated protein Tau and caused alterations of microtubule network in cells. It is noteworthy that paclitaxel combined with thiamet-G resulted in more profound perturbations on microtubule stability than did either one alone, which may implicate the underlying mechanism of thiamet-G-mediated sensitization of leukemia cells to paclitaxel. These findings thus suggest that a regimen of paclitaxel combined with OGA inhibitor might be more effective for the treatment of human leukemia.
A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo.[Pubmed:18587388]
Nat Chem Biol. 2008 Aug;4(8):483-90.
Pathological hyperphosphorylation of the microtubule-associated protein tau is characteristic of Alzheimer's disease (AD) and the associated tauopathies. The reciprocal relationship between phosphorylation and O-GlcNAc modification of tau and reductions in O-GlcNAc levels on tau in AD brain offers motivation for the generation of potent and selective inhibitors that can effectively enhance O-GlcNAc in vertebrate brain. We describe the rational design and synthesis of such an inhibitor (thiamet-G, K(i) = 21 nM; 1) of human O-GlcNAcase. Thiamet-G decreased phosphorylation of tau in PC-12 cells at pathologically relevant sites including Thr231 and Ser396. Thiamet-G also efficiently reduced phosphorylation of tau at Thr231, Ser396 and Ser422 in both rat cortex and hippocampus, which reveals the rapid and dynamic relationship between O-GlcNAc and phosphorylation of tau in vivo. We anticipate that thiamet-G will find wide use in probing the functional role of O-GlcNAc in vertebrate brain, and it may also offer a route to blocking pathological hyperphosphorylation of tau in AD.


