AZD2014Novel mTOR inhibitor CAS# 1009298-59-2 |
- WYE-354
Catalog No.:BCC1059
CAS No.:1062169-56-5
- GDC-mTOR inhibitor
Catalog No.:BCC1781
CAS No.:1207358-59-5
- GDC-0349
Catalog No.:BCC1094
CAS No.:1207360-89-1
- LY 303511
Catalog No.:BCC1715
CAS No.:154447-38-8
- Nordihydroguaiaretic acid
Catalog No.:BCC1805
CAS No.:500-38-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1009298-59-2 | SDF | Download SDF |
| PubChem ID | 25262792 | Appearance | Powder |
| Formula | C25H30N6O3 | M.Wt | 462.54 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Vistusertib | ||
| Solubility | DMSO : ≥ 50 mg/mL (108.10 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
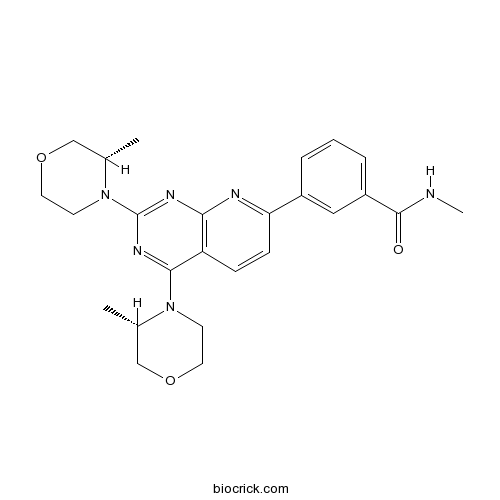
| Chemical Name | 3-[2,4-bis[(3S)-3-methylmorpholin-4-yl]pyrido[2,3-d]pyrimidin-7-yl]-N-methylbenzamide | ||
| SMILES | CC1COCCN1C2=NC(=NC3=C2C=CC(=N3)C4=CC(=CC=C4)C(=O)NC)N5CCOCC5C | ||
| Standard InChIKey | JUSFANSTBFGBAF-IRXDYDNUSA-N | ||
| Standard InChI | InChI=1S/C25H30N6O3/c1-16-14-33-11-9-30(16)23-20-7-8-21(18-5-4-6-19(13-18)24(32)26-3)27-22(20)28-25(29-23)31-10-12-34-15-17(31)2/h4-8,13,16-17H,9-12,14-15H2,1-3H3,(H,26,32)/t16-,17-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | AZD2014 is a novel inhibitor of mTOR with an IC50 value of 2.8 nM. | |||||
| Targets | mTOR | P-Akt (S473) | pS6 (S235/236) | |||
| IC50 | 2.8 nM | 80 nM | 200 nM | |||

AZD2014 Dilution Calculator

AZD2014 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.162 mL | 10.8099 mL | 21.6198 mL | 43.2395 mL | 54.0494 mL |
| 5 mM | 0.4324 mL | 2.162 mL | 4.324 mL | 8.6479 mL | 10.8099 mL |
| 10 mM | 0.2162 mL | 1.081 mL | 2.162 mL | 4.324 mL | 5.4049 mL |
| 50 mM | 0.0432 mL | 0.2162 mL | 0.4324 mL | 0.8648 mL | 1.081 mL |
| 100 mM | 0.0216 mL | 0.1081 mL | 0.2162 mL | 0.4324 mL | 0.5405 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AZD2014 is a novel, potent and highly selective dual inhibitor of the mammalian rapamycin (mTORC1 and mTORC2) with an IC50 value of 2.8 nM. It is an oral inhibitor and possesses potential antineoplastic activity.
AZD2014 enhanced susceptibility of glioblastoma stem-like cells (GSCs) to irradiation both in vitro and under orthotopic in vivo conditions. Kahn J et al pretreated CD133+ and CD15+ GSC cells with AZD2014 (2 µM) for 1 hour, followed by irradiation. The effect was then measured by clonogenic survival analysis [2]. Using in vitro screening, they demonstrated that the combination of ibrutinib, an inhibitor of the tyrosine kinase BTK, and AZD2014 could dramatically induce apoptosis in ABC-subtype DLBCL cell lines. Thereby, the combination of AZD2014 with a BTK inhibitor is a promising therapeutic method to cure patients with ABC-type DLBCL [3]. In hepatocellular carcinoma cells, AZD2014 gave rise to a more complete inhibition of mTORC1 than rapamycin, while the inhibition of mTORC2 prevented the feedback activation of AKT signaling. Therefore, AZD2014 was identified to be more efficacious in the induction of apoptosis, autophagy, and cell cycle arrest, resulting in a significant proliferation suppression of the cells, in contrast with rapamycin [4].
In a recent human pharmacokinetic and pharmacodynamic study, a dose of 50mg BD(twice a day)AZD2014 was recommended to achieve pharmacologically relevant plasma concentrations [5].
References:
Optimization of potent and selective dual mTORC1 and mTORC2 inhibitors: the discovery of AZD8055 and AZD2014. Bioorg Med Chem Lett. 2013 Mar 1;23(5):1212-6.
The mTORC1/mTORC2 inhibitor AZD2014 enhances the radiosensitivity of glioblastoma stem-like cells. Neuro Oncol. 2014 Jan;16(1):29-37.
Synergistic induction of apoptosis by combination of BTK and dual mTORC1/2 inhibitors in diffuse large B cell lymphoma. Oncotarget. 2014 Jul 15;5(13):4990-5001.
Dramatic antitumor effects of the dual mTORC1 and mTORC2 inhibitor AZD2014 in hepatocellular carcinoma. Am J Cancer Res. 2014 Dec 15;5(1):125-39. eCollection 2015.
First-in-human pharmacokinetic and pharmacodynamic study of the dual m-TORC 1/2 inhibitor, AZD2014. Clin Cancer Res. 2015 Mar 24. pii: clincanres.2422.2014.
- AZD8055
Catalog No.:BCC3629
CAS No.:1009298-09-2
- Panamycin 607
Catalog No.:BCN1813
CAS No.:100905-89-3
- Piceatannol
Catalog No.:BCN5824
CAS No.:10083-24-6
- (RS)-CPP
Catalog No.:BCC6561
CAS No.:100828-16-8
- 4,4'-Bis(α,α-dimethylbenzyl)diphenylamine
Catalog No.:BCC8661
CAS No.:10081-67-1
- Chlorahololide C
Catalog No.:BCN7256
CAS No.:1007859-25-7
- (R)-5-Hydroxy-1,7-diphenyl-3-heptanone
Catalog No.:BCN3591
CAS No.:100761-20-4
- Ganoderic acid M
Catalog No.:BCN2871
CAS No.:100761-17-9
- 1-O-Deacetylkhayanolide E
Catalog No.:BCN5823
CAS No.:1007387-95-2
- CH5132799
Catalog No.:BCC4991
CAS No.:1007207-67-1
- 3-(Hydroxymethyl)cyclopentanol
Catalog No.:BCN5822
CAS No.:1007125-14-5
- Deacetyl ganoderic acid F
Catalog No.:BCN2870
CAS No.:100665-44-9
- Caulophyllumine A
Catalog No.:BCN7928
CAS No.:1009318-60-8
- Stachyose
Catalog No.:BCN2566
CAS No.:10094-58-3
- 2,2-Bis(hydroxymethyl)butyric acid
Catalog No.:BCC8495
CAS No.:10097-02-6
- Rotundine
Catalog No.:BCN5983
CAS No.:10097-84-4
- Ebrotidine
Catalog No.:BCC1542
CAS No.:100981-43-9
- Thiamet G
Catalog No.:BCC4864
CAS No.:1009816-48-1
- NSC 687852 (b-AP15)
Catalog No.:BCC2389
CAS No.:1009817-63-3
- CX-4945 (Silmitasertib)
Catalog No.:BCC3693
CAS No.:1009820-21-6
- Levofloxacin
Catalog No.:BCC4791
CAS No.:100986-85-4
- CY 208-243
Catalog No.:BCC6991
CAS No.:100999-26-6
- Pyridostigmine Bromide
Catalog No.:BCC4579
CAS No.:101-26-8
- Hyoscyamine
Catalog No.:BCN1946
CAS No.:101-31-5
Rapamycin-insensitive companion of mTOR (RICTOR) amplification defines a subset of advanced gastric cancer and is sensitive to AZD2014-mediated mTORC1/2 inhibition.[Pubmed:28028034]
Ann Oncol. 2017 Mar 1;28(3):547-554.
Background: Targeting oncogenic genomic aberrations is an established therapeutic strategy in multiple tumor types. Molecular classification has uncovered a number of novel targets, and rapamycin-insensitive companion of mTOR (RICTOR) amplification has been identified in lung cancer. Further investigation assessing the therapeutic potential of RICTOR amplification as a novel target across advanced cancers is needed. Patients and methods: Tumor samples from 640 patients with metastatic solid tumors, primarily gastrointestinal and lung cancers were prospectively subjected to a next-generation sequencing (NGS) assay to identify molecular targets. Samples with NGS-detected RICTOR amplification were confirmed with FISH. A RICTOR-amplified patient-derived cell (PDC) line was generated and used to investigate the effectiveness of selective AKT, mTORC1, and mTORC1/2 inhibition. Results: NGS identified 13 (2%) of 640 patients with RICTOR-amplified tumors (6 gastric, 3 NSCLC, 1 SCLC, 1 CRC, 1 sarcoma, 1 MUO). Of the 13 patients, seven patients had RICTOR protein overexpression by IHC. The prevalence of RICTOR amplification in gastric cancer by NGS was 3.8% (6/160). FISH testing confirmed amplification (RICTOR/control >2) in 5/13 (38%) of samples, including four gastric cancers and one lung cancer. Treatment of a RICTOR amplified PDC with a selective AKT (AZD5363), selective mTORC1 (everolimus), dual mTORC1/2 (AZD2014), and the multi-target kinase inhibitor pazopanib demonstrated preferential sensitivity to the mTORC1/2 inhibitor (AZD2014). Knockdown of RICTOR reversed PDC sensitivity to AZD2014, validating the importance of RICTOR amplification to the PDC line. Conclusions: RICTOR amplification is a rare but therapeutically relevant genomic alteration across solid tumors. Our results support further pre-clinical and clinical investigation with AZD2014 in RICTOR amplified gastric cancer and highlights the importance of genomic profiling.
A Randomised Phase 2 Study of AZD2014 Versus Everolimus in Patients with VEGF-Refractory Metastatic Clear Cell Renal Cancer.[Pubmed:26364551]
Eur Urol. 2016 Mar;69(3):450-6.
BACKGROUND: Everolimus is a mammalian target of rapamycin (mTOR) inhibitor used in vascular endothelial growth factor (VEGF)-refractory metastatic renal cell carcinoma (mRCC). It acts on only part of the mTOR complex (TORC1 alone). In vitro data support the use of mTOR inhibitors with broader activity (TORC1 and TORC2). OBJECTIVE: The purpose of this study was to determine whether combined TORC1 and TORC2 inhibition with AZD2014 has superior activity to everolimus in VEGF-refractory clear cell mRCC. DESIGN, SETTING, AND PARTICIPANTS: Patients with measurable mRCC and VEGF-refractory disease were eligible for this trial. INTERVENTION: Starting in February 2013, patients were randomised (1:1) to AZD2014 (50 mg twice daily) or everolimus (10 mg once daily) until progression of disease at 10 centres across the United Kingdom. OUTCOME MEASUREMENTS AND STATISTICAL ANALYSIS: Progression-free survival (PFS) was the primary end point and was compared using the stratified log-rank test. Secondary end points included tolerability, response rates, overall survival (OS), and pharmacokinetics (PK) analysis. The study was planned to recruit 120 patients. RESULTS AND LIMITATIONS: Recruitment into the trial was stopped early (June 2014) due to lack of efficacy of AZD2014. At that point, 49 patients were randomised (26 to AZD2014 and 23 to everolimus). The PFS for AZD2014 and everolimus was 1.8 and 4.6 mo, respectively (hazard ratio: 2.8 [95% confidence interval (CI), 1.2-6.5]; p=0.01). Progression of disease as the best response to therapy was 69% for AZD2014 and 13% for everolimus (p<0.001). Grade 3-4 adverse events (AEs) occurred in 35% of AZD2014 and 48% of everolimus patients (p=0.3). Only 4% of patients stopped AZD2014 due to AEs. PK analysis suggested concentrations of AZD2014 were compatible with the therapeutic range. Final stratified OS hazard ratio at the time of trial closure (January 2015) was 3.1 (95% CI, 1.1-8.4; p<0.02). CONCLUSIONS: The PFS and OS of AZD2014 were inferior to everolimus in this setting despite acceptable AE and PK profiles. PATIENT SUMMARY: There is a strong rationale for testing mTOR inhibitors with a broader spectrum of activity than everolimus in metastatic clear cell renal cell carcinoma. AZD2014 is such an agent, but in this study, it was inferior to everolimus despite its attractive toxicity profile.
AZD2014 Radiosensitizes Oral Squamous Cell Carcinoma by Inhibiting AKT/mTOR Axis and Inducing G1/G2/M Cell Cycle Arrest.[Pubmed:27031247]
PLoS One. 2016 Mar 31;11(3):e0151942.
BACKGROUND: Oral squamous cell carcinoma (OSCC) is one of the most common malignant neoplasms in Taiwan. Activation of the mTOR signaling pathway has been linked to decreased radiation responsiveness in human oral cancer, thus it limits efficacy of radiotherapy. To address this question, we investigated the effect of AZD2014, a novel small molecular ATP-competitive inhibitor of mTORC1 and mTORC2 kinase, as a radiosensitizer in primary OSCC and OSCC-derived cell line models. METHODS: We isolated primary tumor cells from OSCC tissues and cell lines. AZD2014 was administered with and without ionizing radiation. The radiosensitizing effect of AZD2014 were then assessed using cell viability assays, clonogenic survival assays, and cell cycle analyses. Western blotting was used to detect protein expression. RESULTS: Combination treatment with AZD2014 and irradiation resulted in significant reduction in OSCC cell line and primary OSCC cell colony formation due to the enhanced inhibition of AKT and both mTORC1 and mTORC2 activity. Pre-treatment with AZD2014 in irradiated oral cancer cells induced tumor cell cycle arrest at the G1 and G2/M phases, which led to disruption of cyclin D1-CDK4 and cyclin B1-CDC2 complexes. Moreover, AZD2014 synergized with radiation to promote both apoptosis and autophagy by increasing caspase-3 and LC3 in primary OSCC cells. CONCLUSIONS: These findings suggest that in irradiated OSCC cells, co-treatment with AZD2014, which targets mTORC1 and mTORC2 blockade, is an effective radiosensitizing strategy for oral squamous cell carcinoma.
The novel combination of dual mTOR inhibitor AZD2014 and pan-PIM inhibitor AZD1208 inhibits growth in acute myeloid leukemia via HSF pathway suppression.[Pubmed:26473447]
Oncotarget. 2015 Nov 10;6(35):37930-47.
Mammalian target of rapamycin (mTOR) signaling is a critical pathway in the biology of acute myeloid leukemia (AML). Proviral integration site for moloney murine leukemia virus (PIM) serine/threonine kinase signaling takes part in various pathways exerting tumorigenic properties. We hypothesized that the combination of a PIM kinase inhibitor with an mTOR inhibitor might have complementary growth-inhibitory effects against AML. The simultaneous inhibition of the PIM kinase by pan-PIM inhibitor AZD1208 and of mTOR by selective mTORC1/2 dual inhibitor AZD2014 exerted anticancer properties in AML cell lines and in cells derived from primary AML samples with or without supportive stromal cell co-culture, leading to suppressed proliferation and increased apoptosis. The combination of AZD1208 and AZD2014 rapidly activated AMPKalpha, a negative regulator of translation machinery through mTORC1/2 signaling in AML cells; profoundly inhibited AKT and 4EBP1 activation; and suppressed polysome formation. Inhibition of both mTOR and PIM counteracted induction of heat-shock family proteins, uncovering the master negative regulation of heat shock factor 1 (HSF1), the dominant transcription factor controlling cellular stress responses. The novel combination of the dual mTOR inhibitor and pan-PIM inhibitor synergistically inhibited AML growth by effectively reducing protein synthesis through heat shock factor pathway suppression.


