LY 303511MTOR inhibitor CAS# 154447-38-8 |
- WYE-354
Catalog No.:BCC1059
CAS No.:1062169-56-5
- GDC-mTOR inhibitor
Catalog No.:BCC1781
CAS No.:1207358-59-5
- GDC-0349
Catalog No.:BCC1094
CAS No.:1207360-89-1
- QL-IX-55
Catalog No.:BCC1876
CAS No.:1223002-54-7
- Nordihydroguaiaretic acid
Catalog No.:BCC1805
CAS No.:500-38-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 154447-38-8 | SDF | Download SDF |
| PubChem ID | 3971 | Appearance | Powder |
| Formula | C19H18N2O2 | M.Wt | 306.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1eq. HCl and to 100 mM in DMSO | ||
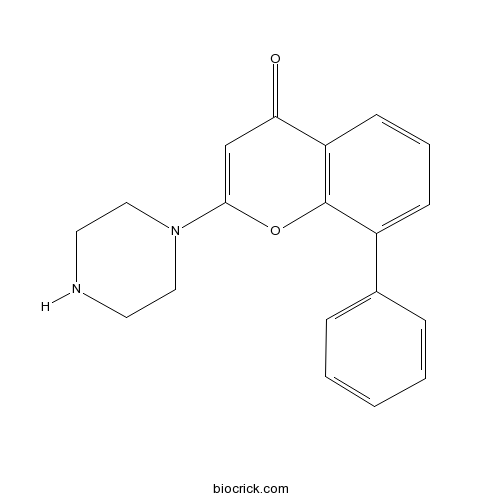
| Chemical Name | 8-phenyl-2-piperazin-1-ylchromen-4-one | ||
| SMILES | C1CN(CCN1)C2=CC(=O)C3=C(O2)C(=CC=C3)C4=CC=CC=C4 | ||
| Standard InChIKey | NGAGMBNBKCDCDJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H18N2O2/c22-17-13-18(21-11-9-20-10-12-21)23-19-15(7-4-8-16(17)19)14-5-2-1-3-6-14/h1-8,13,20H,9-12H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Negative control compound with respect to LY 294002 PI 3-kinase inhibitory activity. Blocks voltage-gated potassium (Kv) channels (IC50 = 64.6 μM) and inhibits IL-1β-stimulated NF-κB activation, attenuating MCP-1 expression. Antiproliferative. Also inhibits the BET bromodomain proteins BRD2, BRD3 and BRD4. |

LY 303511 Dilution Calculator

LY 303511 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2641 mL | 16.3207 mL | 32.6413 mL | 65.2827 mL | 81.6033 mL |
| 5 mM | 0.6528 mL | 3.2641 mL | 6.5283 mL | 13.0565 mL | 16.3207 mL |
| 10 mM | 0.3264 mL | 1.6321 mL | 3.2641 mL | 6.5283 mL | 8.1603 mL |
| 50 mM | 0.0653 mL | 0.3264 mL | 0.6528 mL | 1.3057 mL | 1.6321 mL |
| 100 mM | 0.0326 mL | 0.1632 mL | 0.3264 mL | 0.6528 mL | 0.816 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value:N/A LY303511, an inactive analogue of LY294002, is a mTOR inhibitor that did not inhibit PI3-K. in vitro: 100 μM LY303511 significantly reduced the fraction of cells in S phase. The proportion of cells in G2/M remained unchanged, indicating that cells were arrested in both G1 and G2/M. In contrast, rapamycin increased the G1 population by reducing the proportion of cells in both S and G2/M. The effects of 10 μM LY303511 and rapamycin on the reduction in S phase cells were additive to that of 10 μM LY303511 alone (P = 0.056) [1]. In MIN6 insulinoma cells, wortmannin (100 nM) had no effect on whole-cell outward K+ currents, but LY294002 and LY303511 reversibly blocked currents in a dose-dependent manner (IC50=9.0+/-0.7 microM and 64.6+/-9.1 microM, respectively). Western blotting confirmed the specific inhibitory effects of LY294002 and wortmannin on insulin-stimulated PI3K activity [2]. Both LY294002 and LY303511 increased the activity of protein kinase A (PKA). Moreover, PKA blockade by the small molecule inhibitor H89 decreased the LY294002/LY303511-mediated increase in GJIC [3]. in vivo: PND4 ovaries were cultured for 8 days in control medium or medium containing VCD (30 μM) in the presence or absence of LY303511 (20 μM). Incubation with LY303511 alone caused a reduction (P < 0.05) in primordial and small primary follicle numbers. On the other hand, whereas VCD alone depleted (P < 0.05) primordial and small primary follicle numbers, this depletion was not prevented by co-incubation with LY303511 [4]. Clinical trial: N/A
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- NU 7026
Catalog No.:BCC3933
CAS No.:154447-35-5
- Fmoc-Arg(Pbf)-OH
Catalog No.:BCC3040
CAS No.:154445-77-9
- Pramanicin
Catalog No.:BCN1853
CAS No.:154445-05-3
- Lysicamine
Catalog No.:BCN6523
CAS No.:15444-20-9
- Zinc protoporphyrin IX
Catalog No.:BCC6775
CAS No.:15442-64-5
- 5,5'-Dimethoxylariciresinol 4-O-glucoside
Catalog No.:BCN1556
CAS No.:154418-16-3
- Methyl 3-O-feruloylquinate
Catalog No.:BCN3403
CAS No.:154418-15-2
- Capecitabine
Catalog No.:BCN2168
CAS No.:154361-50-9
- Echistatin, α1 isoform
Catalog No.:BCC5988
CAS No.:154303-05-6
- CB-5083
Catalog No.:BCC6528
CAS No.:1542705-92-9
- Ampalex
Catalog No.:BCC1359
CAS No.:154235-83-3
- Methyl 5-O-feruloylquinate
Catalog No.:BCN3402
CAS No.:154461-64-0
- Sinapaldehyde glucoside
Catalog No.:BCN1689
CAS No.:154461-65-1
- CHM 1
Catalog No.:BCC2387
CAS No.:154554-41-3
- SB 204990
Catalog No.:BCC6342
CAS No.:154566-12-8
- Stauprimide
Catalog No.:BCC7768
CAS No.:154589-96-5
- Efavirenz
Catalog No.:BCC4135
CAS No.:154598-52-4
- Tezampanel
Catalog No.:BCC1993
CAS No.:154652-83-2
- 3-Methoxymollugin
Catalog No.:BCN7164
CAS No.:154706-44-2
- 2-(2'-Hydroxytetracosanoylamino)-octadecane-1,3,4-triol
Catalog No.:BCN1555
CAS No.:154801-30-6
- Cimiside E
Catalog No.:BCN7951
CAS No.:154822-57-8
- 4-Acetoxycinnamic acid
Catalog No.:BCN5026
CAS No.:15486-19-8
- Eleutheroside C
Catalog No.:BCN1690
CAS No.:15486-24-5
Clinical and immunohistochemical performance of lyophilized platelet-rich fibrin (Ly-PRF) on tissue regeneration.[Pubmed:28192870]
Clin Implant Dent Relat Res. 2017 Jun;19(3):466-477.
BACKGROUND: Platelet-rich fibrin (PRF) has been widely used in oral implantology and other fields, but benefits of the fresh PRF (FPRF (fresh platelet-rich fibrin)) were consequently limited because of its short-term application. Thus, a protocol for the combination of PRF and lyophilization comes up in the present study to address the issue of PRF storage and delayed clinical application, which has little been reported in this field at home and abroad by now. PURPOSE: The aim of the present study was to evaluate the applicability of lyophilized platelet-rich fibrin (Ly-PRF) used as the scaffold material for craniofacial tissue regeneration and to compare its biochemical properties with commonly used fresh PRF. MATERIALS AND METHODS: Two volunteers with both genders were selected as the source of PRF and Ly-PRF samples. Macro- and micro-scopic appearance evaluation as well as immunohistochemical comparison were performed on PRF samples before and after freeze-drying at -196 degrees C. The second experimental phase was to observe clinical performance when fresh and lyophilized PRF were applied in guided bone regeneration (GBR) operations in 39 patients losing teeth in the anterior maxillary region who required an oral implantation followed by labial bone grafting. RESULTS: The conventional histological and transmission electron microscopy images showed the microstructure of Ly-PRF, which resembled a mesh containing apparently irregularly shaped platelets with less alpha-granule than fresh PRF in micro and a translucent membrane with less elasticity than fresh PRF in macro. Simultaneous immunohistological staining results showed positive expression of PDGF-BB, IL-1, IL-4, TNF, TGF-beta1 in both fresh and lyophilized PRF, while the expression of PDGF-BB, IL-1, TNF, TGF-beta1 has no statistical difference between them (P > .05) but that of IL-4 in Ly-PRF is statistically higher than in fresh PRF (P < .05). When applied in GBR operations, there were no significant differences between Ly-PRF and FPRF in factors of histological and clinical evaluations (i.e., color, swelling, bleeding of the mucosa, pain leveland, and remodeling of hard tissue) performed 3 days, 7 days, and 4 months after the surgery (P > .05). CONCLUSIONS: This study strongly supports that lyophilization at -196 degrees C does not largely influence the expression of bioactive factors, the microstructure of fibrinogen or the clinical effects of PRF.
Expansion of CD11b(+)Ly-6C(+) myeloid-derived suppressor cells (MDSCs) driven by galectin-9 attenuates CVB3-induced myocarditis.[Pubmed:28110209]
Mol Immunol. 2017 Mar;83:62-71.
Galectin-9 is known to play a role in the modulation of innate and adaptive immunity to ameliorate CVB3-induced myocarditis. In the present study, we found that galectin-9 induced the expansion of CD11b(+)Ly-6C(+) myeloid-derived suppressor cells (MDSCs) in the heart from CVB3-infected mice. Adoptive transfer of CD11b(+)Ly-6C(+) MDSCs significantly alleviated myocarditis accompanied by increased Th2 and Treg frequency and anti-inflammatory cytokines expression in the heart tissue. Moreover, Ly6C(+) MDSCs, but not Ly6G(+) cells, expressed Arg-1 and NOS2, and suppressed CD4(+) T cell proliferation in vitro in an Arg-1-dependent mechanism; an event that was reversed with treatment of either an Arg-1 inhibitor or addition of excess l-arginine. Furthermore, Ly6C(+) MDSCs co-expressed higher levels of F4/80, Tim-3, and IL-4Ralpha, and had the plasticity to up-regulate NOS2 or Arg-1 in response to IFN-gamma or IL-4 treatment. The present results indicate that galectin-9 expands CD11b(+)Ly-6C(+) MDSCs to ameliorate CVB3-induced myocarditis.
Assessment of roles for the Rho-specific guanine nucleotide dissociation inhibitor Ly-GDI in platelet function: a spatial systems approach.[Pubmed:28148498]
Am J Physiol Cell Physiol. 2017 Apr 1;312(4):C527-C536.
On activation at sites of vascular injury, platelets undergo morphological alterations essential to hemostasis via cytoskeletal reorganizations driven by the Rho GTPases Rac1, Cdc42, and RhoA. Here we investigate roles for Rho-specific guanine nucleotide dissociation inhibitor proteins (RhoGDIs) in platelet function. We find that platelets express two RhoGDI family members, RhoGDI and Ly-GDI. Whereas RhoGDI localizes throughout platelets in a granule-like manner, Ly-GDI shows an asymmetric, polarized localization that largely overlaps with Rac1 and Cdc42 as well as microtubules and protein kinase C (PKC) in platelets adherent to fibrinogen. Antibody interference and platelet spreading experiments suggest a specific role for Ly-GDI in platelet function. Intracellular signaling studies based on interactome and pathways analyses also support a regulatory role for Ly-GDI, which is phosphorylated at PKC substrate motifs in a PKC-dependent manner in response to the platelet collagen receptor glycoprotein (GP) VI-specific agonist collagen-related peptide. Additionally, PKC inhibition diffuses the polarized organization of Ly-GDI in spread platelets relative to its colocalization with Rac1 and Cdc42. Together, our results suggest a role for Ly-GDI in the localized regulation of Rho GTPases in platelets and hypothesize a link between the PKC and Rho GTPase signaling systems in platelet function.
Short-term dabigatran interruption before cardiac rhythm device implantation: multi-centre experience from the RE-LY trial.[Pubmed:28339794]
Europace. 2017 Oct 1;19(10):1630-1636.
Aims: Cardiac implantable electronic device (CIED) surgery is commonly performed in patients with atrial fibrillation (AF). The current analysis was undertaken to compare peri-operative anticoagulation management, bleeding, and thrombotic events in AF patients treated with dabigatran vs. warfarin. Methods and results: This study included 611 patients treated with dabigatran vs. warfarin who underwent CIED surgery during the RE-LY trial. Among 201 warfarin-treated patients, warfarin was interrupted a median of 144 (inter-quartile range, IQR: 120-216) h, and 37 (18.4%) patients underwent heparin bridging. In dabigatran-treated patients (216 on 110 mg bid and 194 on 150 mg bid), the duration of dabigatran interruption was a median of 96 (IQR: 61-158) h. Pocket hematomas occurred in 9 (2.20%) patients on dabigatran and 8 (3.98%) patients on warfarin (P = 0.218). The occurrence of pocket hematomas was lower with dabigatran compared with warfarin with heparin bridging (RD: -8.62%, 95% CI: -24.15 to - 0.51%, P = 0.034) but not when compared with warfarin with no bridging (P = 0.880). Ischemic stroke occurred in 2 (0.3%) patients; one in the warfarin group (without bridging) and one in the dabigatran 150 mg bid group (P = 0.735). Conclusion: In patients treated with dabigatran undergoing CIED surgery, interruption of dabigatran is associated with similar or lower incidence of pocket hematoma, when compared with warfarin interruption without or with heparin bridging, respectively. Whether uninterrupted dabigatran can reduce pocket hematoma or ischemic stroke remains to be evaluated.
LY303511 (2-piperazinyl-8-phenyl-4H-1-benzopyran-4-one) acts via phosphatidylinositol 3-kinase-independent pathways to inhibit cell proliferation via mammalian target of rapamycin (mTOR)- and non-mTOR-dependent mechanisms.[Pubmed:15923340]
J Pharmacol Exp Ther. 2005 Sep;314(3):1134-43.
Mammalian target of rapamycin (mTOR), a serine/threonine kinase, regulates cell growth and proliferation in part via the activation of p70 S6 kinase (S6K). Rapamycin is an antineo-plastic agent that, in complex with FKBP12, is a specific inhibitor of mTOR through interaction with its FKBP12-rapamycin binding domain, thereby causing G(1) cell cycle arrest. However, cancer cells often develop resistance to rapamycin, and alternative inhibitors of mTOR are desired. 2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) blocks mTOR kinase activity, but it also inhibits phosphatidylinositol 3-kinase (PI3K), an enzyme that regulates cellular functions other than proliferation. We hypothesized that a close structural analog, 2-piperazinyl-8-phenyl-4H-1-benzopyran-4-one (LY303511) might inhibit mTOR-dependent cell proliferation without unwanted effects on PI3K. In human lung epithelial adenocarcinoma (A549) cells, LY303511, like rapamycin, inhibited mTOR-dependent phosphorylation of S6K, but not PI3K-dependent phosphorylation of Akt. LY303511 blocked proliferation in A549 as well as in primary pulmonary artery smooth muscle cells, without causing apoptosis. In contrast to rapamycin, LY303511 reduced G(2)/M progression as well as G(2)/M-specific cyclins in A549 cells. Consistent with an additional mTOR-independent kinase target, LY303511 inhibited casein kinase 2 activity, a known regulator of G(1) and G(2)/M progression. In addition to its antiproliferative effect in vitro, LY303511 inhibited the growth of human prostate adenocarcinoma tumor implants in athymic mice. Given its inhibition of cell proliferation via mTOR-dependent and independent mechanisms, LY303511 has therapeutic potential with antineoplastic actions that are independent of PI3K inhibition.
LY294002 inhibits monocyte chemoattractant protein-1 expression through a phosphatidylinositol 3-kinase-independent mechanism.[Pubmed:14960322]
FEBS Lett. 2004 Feb 13;559(1-3):141-4.
The effects of LY294002 (LY29) and wortmannin (WM), inhibitors of phosphatidylinositol 3-kinase (PI3K), on monocyte chemoattractant protein-1 (MCP-1) expression by human umbilical vein endothelial cells were investigated. Complete inhibition of interleukin (IL)-1beta-induced Akt phosphorylation occurred at 50 microM LY29 or 100 nM WM. At these concentrations, LY29, but not WM, significantly inhibited constitutive and IL-1beta-induced MCP-1 expression at both protein and mRNA levels. LY303511 (LY30), an inactive analogue of LY29, also inhibited MCP-1 expression. LY29 and LY30 inhibited activation of nuclear factor-kappaB (NF-kappaB). These results suggest that LY29 inhibits MCP-1 expression at least in part via suppression of NF-kappaB, independent of PI3K, and the structure of LY29 and LY30 may be a novel template for development of new anti-inflammatory drugs.
The phosphatidylinositol 3-kinase inhibitor LY294002 potently blocks K(V) currents via a direct mechanism.[Pubmed:12586735]
FASEB J. 2003 Apr;17(6):720-2.
Voltage-dependent K+ (Kv) channels negatively regulate Ca2+ entry into pancreatic beta-cells by repolarizing glucose-stimulated action potentials. A role for phosphatidylinositol 3-kinase (PI3K) modulation of Kv channel function was investigated using the PI3K inhibitors wortmannin and LY294002, and LY303511, a negative control compound with respect to PI3K activity. In MIN6 insulinoma cells, wortmannin (100 nM) had no effect on whole-cell outward K+ currents, but LY294002 and LY303511 reversibly blocked currents in a dose-dependent manner (IC50=9.0+/-0.7 microM and 64.6+/-9.1 microM, respectively). Western blotting confirmed the specific inhibitory effects of LY294002 and wortmannin on insulin-stimulated PI3K activity. Kv currents in rat beta-cells at near physiological temperatures were inhibited 92% by 25 microM LY294002. Kv2.1 and Kv1.4 are highly expressed in beta-cells, and in Kv2.1-transfected tsA201 cells, 50 microM LY294002 and 100 microM LY303511 reversibly inhibited currents by 99% and 41%, respectively. In Kv1.4-transfected tsA201 cells, 50 microM LY294002 reduced the inactivation time constant from 73 to 18 ms. The insulinotropic properties of LY294002 and its effects in other excitable cells may be caused by inhibition of Kv currents rather than PI3K antagonism. Furthermore, LY294002 may represent a novel structure from which future Kv channel blockers may be developed.


