PramanicinCAS# 154445-05-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 154445-05-3 | SDF | Download SDF |
| PubChem ID | 11810750 | Appearance | Cryst. |
| Formula | C19H31NO6 | M.Wt | 369.46 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
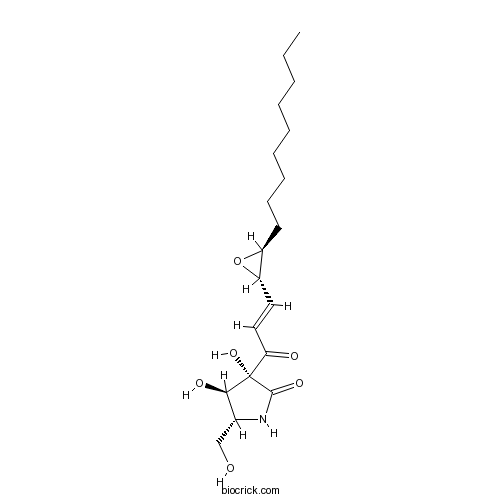
| Chemical Name | (3R,4R,5R)-3,4-dihydroxy-5-(hydroxymethyl)-3-[(E)-3-[(2S,3S)-3-nonyloxiran-2-yl]prop-2-enoyl]pyrrolidin-2-one | ||
| SMILES | CCCCCCCCCC1C(O1)C=CC(=O)C2(C(C(NC2=O)CO)O)O | ||
| Standard InChIKey | BOWRHOKHYKPEAR-DUNGTTFGSA-N | ||
| Standard InChI | InChI=1S/C19H31NO6/c1-2-3-4-5-6-7-8-9-14-15(26-14)10-11-16(22)19(25)17(23)13(12-21)20-18(19)24/h10-11,13-15,17,21,23,25H,2-9,12H2,1H3,(H,20,24)/b11-10+/t13-,14+,15+,17-,19+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Pramanicin, an antifungal agent, has vasorelaxant effect, it induces a slow endothelium-dependent relaxation, which could be reversed with the NO synthase inhibitor, L-NOARG . 2. Pramanicin has potent, selective, and irreversible inhibitory effect on the endothelial , thus it has potential development into an anti-angiogenic drug. 3. Pramanicin causes an initial slow endothelium-dependent, NO-mediated vascular relaxation, followed by a cytotoxic effect on vascular endothelial cells, eventually resulting in the loss of vasorelaxant function. 4. Pramanicin as a potential apoptosis-inducing small molecule, which acts through a well-defined JNK- and p38-dependent apoptosis signalling pathway in Jurkat T leukemia cells. 5. Pramanicin is an antimicrobial agent . |
| Targets | Calcium Channel | NO | NOS | Caspase | JNK | ERK | p38MAPK | Antifection |

Pramanicin Dilution Calculator

Pramanicin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7067 mL | 13.5333 mL | 27.0665 mL | 54.1331 mL | 67.6663 mL |
| 5 mM | 0.5413 mL | 2.7067 mL | 5.4133 mL | 10.8266 mL | 13.5333 mL |
| 10 mM | 0.2707 mL | 1.3533 mL | 2.7067 mL | 5.4133 mL | 6.7666 mL |
| 50 mM | 0.0541 mL | 0.2707 mL | 0.5413 mL | 1.0827 mL | 1.3533 mL |
| 100 mM | 0.0271 mL | 0.1353 mL | 0.2707 mL | 0.5413 mL | 0.6767 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lysicamine
Catalog No.:BCN6523
CAS No.:15444-20-9
- Zinc protoporphyrin IX
Catalog No.:BCC6775
CAS No.:15442-64-5
- 5,5'-Dimethoxylariciresinol 4-O-glucoside
Catalog No.:BCN1556
CAS No.:154418-16-3
- Methyl 3-O-feruloylquinate
Catalog No.:BCN3403
CAS No.:154418-15-2
- Capecitabine
Catalog No.:BCN2168
CAS No.:154361-50-9
- Echistatin, α1 isoform
Catalog No.:BCC5988
CAS No.:154303-05-6
- CB-5083
Catalog No.:BCC6528
CAS No.:1542705-92-9
- Ampalex
Catalog No.:BCC1359
CAS No.:154235-83-3
- Abiraterone
Catalog No.:BCC2259
CAS No.:154229-19-3
- Abiraterone Acotate
Catalog No.:BCN2184
CAS No.:154229-18-2
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- N-[2-Isopropylthiazol-4-ylmethyl(methyl)carbamoyl]-L-valine
Catalog No.:BCC9067
CAS No.:154212-61-0
- Fmoc-Arg(Pbf)-OH
Catalog No.:BCC3040
CAS No.:154445-77-9
- NU 7026
Catalog No.:BCC3933
CAS No.:154447-35-5
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- LY 303511
Catalog No.:BCC1715
CAS No.:154447-38-8
- Methyl 5-O-feruloylquinate
Catalog No.:BCN3402
CAS No.:154461-64-0
- Sinapaldehyde glucoside
Catalog No.:BCN1689
CAS No.:154461-65-1
- CHM 1
Catalog No.:BCC2387
CAS No.:154554-41-3
- SB 204990
Catalog No.:BCC6342
CAS No.:154566-12-8
- Stauprimide
Catalog No.:BCC7768
CAS No.:154589-96-5
- Efavirenz
Catalog No.:BCC4135
CAS No.:154598-52-4
- Tezampanel
Catalog No.:BCC1993
CAS No.:154652-83-2
- 3-Methoxymollugin
Catalog No.:BCN7164
CAS No.:154706-44-2
Vasorelaxant effects of pramanicin, an anti-fungal agent: selective action on endothelial cells.[Pubmed:11325015]
Jpn J Pharmacol. 2001 Mar;85(3):234-40.
A newly discovered antifungal agent, Pramanicin, within the therapeutically effective concentration range (4-100 microM), inhibits the tone of phenylephrine (PE)-precontracted dog carotid arterial rings in a concentration-dependent manner and leads to gradual development of relaxation. However, Pramanicin had no effect on rings precontracted with 100 mM KCl or on endothelium-denuded rings. Thus, inhibition by Pramanicin of PE-induced contraction was endothelium-dependent. Preincubation of 100 microM Pramanicin with carotid arterial rings for 30 min did not significantly affect the concentration-contraction response to PE, but almost completely inhibited the endothelium-dependent relaxation response to subsequent addition of 3 microM carbachol or 100 microM Pramanicin. This irreversible inhibition of endothelium-dependent relaxation, which is independent of extracellular Ca2+, suggests possible endothelial cell damage by Pramanicin. Pretreatment of the endothelium-intact vascular rings with L-N(G)-nitro-arginine (100 microM) inhibited the relaxation of PE-precontracted rings induced by 3 microM carbachol or 100 microM Pramanicin, suggesting that the generation of nitric oxide (NO) in endothelial cells mediates the slow vascular relaxation induced by Pramanicin. We conclude that Pramanicin has little direct effect on the contractility of smooth muscle cells, but causes an initial slow endothelium-dependent, NO-mediated vascular relaxation. This is followed by a cytotoxic effect on vascular endothelial cells, eventually resulting in the loss of vasorelaxant function.
Pramanicin, an antifungal agent, raises cytosolic Ca2+ and causes cell death in vascular endothelial cells.[Pubmed:12646408]
Vascul Pharmacol. 2003 Jan;40(1):35-42.
The effects of a newly discovered antifungal agent, Pramanicin, on cytosolic Ca(2+) and cell viability of cultured bovine pulmonary artery endothelial cells and on endothelium-dependent relaxation of dog carotid arterial rings were investigated by digital dynamic fluorescence ratio imaging and morphological and contractility studies, respectively. Pramanicin 100 microM, previously shown to cause maximal endothelium-dependent and NO-mediated vascular relaxation, induced a small transient elevation of cytosolic Ca(2+) concentration in Ca(2+)-free medium; subsequent introduction of 1 mM Ca(2+) caused a steady, nonsaturating increase of Ca(2+), which could be brought down to the basal level by the addition of EGTA. At the single cell level, the elevation of cytosolic Ca(2+) initiates from the cell periphery and progresses toward the central region. When added to the plateau phase of phenylephrine-induced contraction, Pramanicin induced a slow endothelium-dependent relaxation, which could be reversed with the NO synthase inhibitor, L-NOARG. When preincubated with vascular tissue, Pramanicin resulted in an irreversible loss of endothelial function characterized by the lack of carbachol-induced relaxation. Pramanicin caused cell injury characterized by plasmalemmal bleb formation, leading to cell death characterized by Trypan blue staining of the nuclei in cultured vascular endothelial cells in a concentration- and time-dependent manner. Such Pramanicin-induced cell death was not associated with Ca(2+)-mediated or NO-mediated mechanisms. The time course of Ca(2+) elevation corresponds with that of Pramanicin-induced relaxation of precontracted arterial rings, whereas the time course of endothelial cell death corresponds to that of Pramanicin-induced loss of endothelial function as assessed by carbachol-induced relaxation. The Pramanicin analogue, PMC-A, a by-product of the biosynthesis of Pramanicin, in which the epoxy group is replaced by a CC bond, caused little endothelial-dependent relaxation, but it was able to cause endothelial cell dysfunction, albeit to a lesser extent compared to Pramanicin, suggesting a role of the epoxy group in Pramanicin for its vasorelaxant effect.
Pramanicin induces apoptosis in Jurkat leukemia cells: a role for JNK, p38 and caspase activation.[Pubmed:15909121]
Apoptosis. 2005 May;10(3):597-609.
Pramanicin is a novel anti-fungal drug with a wide range of potential application against human diseases. It has been previously shown that Pramanicin induces cell death and increases calcium levels in vascular endothelial cells. In the present study, we showed that Pramanicin induced apoptosis in Jurkat T leukemia cells in a dose- and time-dependent manner. Our data reveal that Pramanicin induced the release of cytochrome c and caspase-9 and caspase-3 activation, as evidenced by detection of active caspase fragments and fluorometric caspase assays. Pramanicin also activated c-jun N-terminal kinase (JNK), p38 and extracellular signal-regulated kinases (ERK 1/2) with different time and dose kinetics. Treatment of cells with specific MAP kinase and caspase inhibitors further confirmed the mechanistic involvement of these signalling cascades in Pramanicin-induced apoptosis. JNK and p38 pathways acted as pro-apoptotic signalling pathways in Pramanicin-induced apoptosis, in which they regulated release of cytochrome c and caspase activation. In contrast the ERK 1/2 pathway exerted a protective effect through inhibition of cytochrome c leakage from mitochondria and caspase activation, which were only observed when lower concentrations of Pramanicin were used as apoptosis-inducing agent and which were masked by the intense apoptosis induction by higher concentrations of Pramanicin. These results suggest Pramanicin as a potential apoptosis-inducing small molecule, which acts through a well-defined JNK- and p38-dependent apoptosis signalling pathway in Jurkat T leukemia cells.
The epoxy group of pramanicin is required for the optimal endothelium-dependent relaxation of rat aorta.[Pubmed:12890885]
J Pharmacol Sci. 2003 Jul;92(3):203-8.
The vascular effects of a newly discovered anti-fungal agent, Pramanicin (PMC), and its two analogues, PMC-A, in which the epoxy group is replaced by a - HC = CH - bond, and PMC-B, on which the diene is converted to the saturated (CH(2))(4)-derivative, respectively, were investigated in rat aorta. All three compounds caused an initial endothelial-dependent relaxation, which is prevented either by removal of endothelium or inclusion of the nitric oxide synthase inhibitor L-NAME. Upon prolonged incubation with the aortic rings, they also caused endothelial cell dysfunction characterized as reduced relaxation to carbachol (CCh). These effects were the strongest for PMC, being completely inhibitory at 20 microM after 30 min incubation, whereas those of PMC-A and PMC-B were smaller and comparable with each other, causing 30 - 40% inhibition at 20 micro M. PMC and its analogues had no effect on KCl-induced contraction and also had no effect on relaxation induced by sodium nitroprusside, suggesting that these compounds had no effect on the basic mechanisms of the contractile elements. Phenylephrine (PE)-induced contraction, however, was significantly reduced in the presence of these compounds, the inhibitory effect being strongest with PMC, but this inhibitory action was rapidly reversible and not of the competitive mode with respect to PE. We conclude that the epoxy group in PMC is required for the optimal vascular effects. We have discussed and speculated upon the possible mechanisms of action of PMC. The potent, selective, and irreversible inhibitory effect of PMC on the endothelial function points to its potential development into an anti-angiogenic drug.
Synthesis of mimics of pramanicin from pyroglutamic acid and their antibacterial activity.[Pubmed:25647715]
J Org Chem. 2015 Mar 6;80(5):2661-75.
Epoxypyrrolidinones are available by epoxidation of carboxamide-activated bicyclic lactam substrates derived from pyroglutamate using aqueous hydrogen peroxide and tertiary amine catalysis. In the case of an activating Weinreb carboxamide, further chemoselective elaboration leads to the efficient formation of libraries of epoxyketones. Deprotection may be achieved under acidic conditions to give epoxypyroglutaminols, although the ease of this process can be ameliorated by the presence of internal hydrogen bonding. Bioassay against S. aureus and E. coli indicated that some compounds exhibit antibacterial activity. These libraries may be considered to be structural mimics of the natural products Pramanicin and epolactaene. More generally, this outcome suggests that interrogation of bioactive natural products is likely to permit the identification of "privileged" structural scaffolds, providing frameworks suitable for optimization in a short series of chemical steps that may accelerate the discovery of new antibiotic chemotypes. Further optimization of such systems may permit the rapid identification of novel systems suitable for antibacterial drug development.


