CHM 1Tubulin polymerization inhibitor CAS# 154554-41-3 |
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

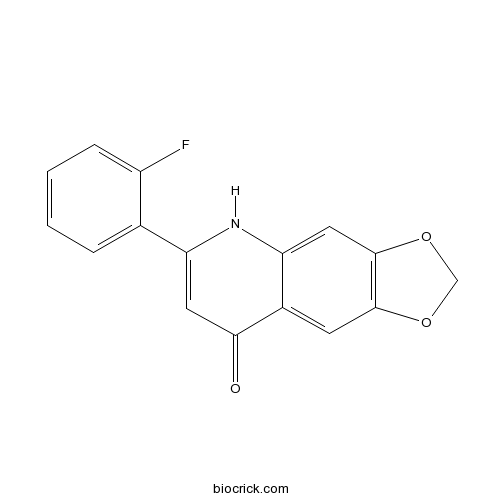
| Cas No. | 154554-41-3 | SDF | Download SDF |
| PubChem ID | 375860 | Appearance | Powder |
| Formula | C16H10FNO3 | M.Wt | 283.25 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in water | ||
| Chemical Name | 6-(2-fluorophenyl)-5H-[1,3]dioxolo[4,5-g]quinolin-8-one | ||
| SMILES | C1OC2=C(O1)C=C3C(=C2)C(=O)C=C(N3)C4=CC=CC=C4F | ||
| Standard InChIKey | ZMYDAPJHGNEFGQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H10FNO3/c17-11-4-2-1-3-9(11)12-6-14(19)10-5-15-16(21-8-20-15)7-13(10)18-12/h1-7H,8H2,(H,18,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inducer of apoptosis; displays potent antitumor ability in human hepatocellular carcinoma. Inhibits tubulin polymerization in vitro and in vivo. Causes cell cycle arrest at G2/M phase by activation of Cdc2 kinase activity. Induces translocation of apoptosis inducing factor (AIF) from the mitochondria to nucleus. Also exhibits vascular targeting activity through upregulation of p53 and induction of death receptor (DR5)-mediated apoptosis in HUVEC cells. | |||||

CHM 1 Dilution Calculator

CHM 1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5305 mL | 17.6523 mL | 35.3045 mL | 70.609 mL | 88.2613 mL |
| 5 mM | 0.7061 mL | 3.5305 mL | 7.0609 mL | 14.1218 mL | 17.6523 mL |
| 10 mM | 0.353 mL | 1.7652 mL | 3.5305 mL | 7.0609 mL | 8.8261 mL |
| 50 mM | 0.0706 mL | 0.353 mL | 0.7061 mL | 1.4122 mL | 1.7652 mL |
| 100 mM | 0.0353 mL | 0.1765 mL | 0.353 mL | 0.7061 mL | 0.8826 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Inducer of apoptosis; displays potent antitumor ability in human hepatocellular carcinoma. Inhibits tubulin polymerization in vitro and in vivo. Causes cell cycle arrest at G2/M phase by activation of Cdc2 kinase activity. Induces translocation of apoptosis inducing factor (AIF) from the mitochondria to nucleus. Also exhibits vascular targeting activity through upregulation of p53 and induction of death receptor (DR5)-mediated apoptosis in HUVEC cells.
- Sinapaldehyde glucoside
Catalog No.:BCN1689
CAS No.:154461-65-1
- Methyl 5-O-feruloylquinate
Catalog No.:BCN3402
CAS No.:154461-64-0
- LY 303511
Catalog No.:BCC1715
CAS No.:154447-38-8
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- NU 7026
Catalog No.:BCC3933
CAS No.:154447-35-5
- Fmoc-Arg(Pbf)-OH
Catalog No.:BCC3040
CAS No.:154445-77-9
- Pramanicin
Catalog No.:BCN1853
CAS No.:154445-05-3
- Lysicamine
Catalog No.:BCN6523
CAS No.:15444-20-9
- Zinc protoporphyrin IX
Catalog No.:BCC6775
CAS No.:15442-64-5
- 5,5'-Dimethoxylariciresinol 4-O-glucoside
Catalog No.:BCN1556
CAS No.:154418-16-3
- Methyl 3-O-feruloylquinate
Catalog No.:BCN3403
CAS No.:154418-15-2
- Capecitabine
Catalog No.:BCN2168
CAS No.:154361-50-9
- SB 204990
Catalog No.:BCC6342
CAS No.:154566-12-8
- Stauprimide
Catalog No.:BCC7768
CAS No.:154589-96-5
- Efavirenz
Catalog No.:BCC4135
CAS No.:154598-52-4
- Tezampanel
Catalog No.:BCC1993
CAS No.:154652-83-2
- 3-Methoxymollugin
Catalog No.:BCN7164
CAS No.:154706-44-2
- 2-(2'-Hydroxytetracosanoylamino)-octadecane-1,3,4-triol
Catalog No.:BCN1555
CAS No.:154801-30-6
- Cimiside E
Catalog No.:BCN7951
CAS No.:154822-57-8
- 4-Acetoxycinnamic acid
Catalog No.:BCN5026
CAS No.:15486-19-8
- Eleutheroside C
Catalog No.:BCN1690
CAS No.:15486-24-5
- 3,5-Dihydroxy-4',7-dimethoxyflavone
Catalog No.:BCN1691
CAS No.:15486-33-6
- Kaempferol 3,7,4'-trimethylether
Catalog No.:BCN4087
CAS No.:15486-34-7
- LL 37
Catalog No.:BCC8027
CAS No.:154947-66-7
Chm-1 gene-modified bone marrow mesenchymal stem cells maintain the chondrogenic phenotype of tissue-engineered cartilage.[Pubmed:27150539]
Stem Cell Res Ther. 2016 May 5;7(1):70.
BACKGROUND: Marrow mesenchymal stem cells (MSCs) can differentiate into specific phenotypes, including chondrocytes, and have been widely used for cartilage tissue engineering. However, cartilage grafts from MSCs exhibit phenotypic alternations after implantation, including matrix calcification and vascular ingrowth. METHODS: We compared chondromodulin-1 (Chm-1) expression between chondrocytes and MSCs. We found that chondrocytes expressed a high level of Chm-1. We then adenovirally transduced MSCs with Chm-1 and applied modified cells to engineer cartilage in vivo. RESULTS: A gross inspection and histological observation indicated that the chondrogenic phenotype of the tissue-engineered cartilage graft was well maintained, and the stable expression of Chm-1 was detected by immunohistological staining in the cartilage graft derived from the Chm-1 gene-modified MSCs. CONCLUSIONS: Our findings defined an essential role for Chm-1 in maintaining chondrogenic phenotype and demonstrated that Chm-1 gene-modified MSCs may be used in cartilage tissue engineering.
A pioneer paediatrician in New Zealand Geoffrey Bruton SWEET (1.9.1870-17.5.1939) MB ChM (Sydney 1893).[Pubmed:27857244]
N Z Med J. 2016 Nov 18;129(1445):110-114.
Paediatrics as a specialty was slow to emerge in New Zealand where Geoffrey Bruton Sweet was a pioneer full-time paediatrician from 1907 to 1939. Although there had been appointments as paediatric lecturers to the Otago Medical School, the early appointees were not restricted to paediatric practice. Dr Sweet, an Australian graduate, came to New Zealand and made a major contribution to the development of pathology services before embarking on a career devoted to paediatrics. He was a powerful advocate for children, well read and interested in clinical research, and realised the need to communicate with those directly involved in the care of children. For New Zealand, he was a man ahead of his time.
Inhibition of the insulin-like growth factor 1 receptor by CHM-1 blocks proliferation of glioblastoma multiforme cells.[Pubmed:25643584]
Chem Biol Interact. 2015 Apr 25;231:119-26.
The insulin-like growth factor-1 receptor (IGF-1R) plays a pivotal role in transformation, growth, and survival of glioblastoma multiforme (GBM) cells, and has emerged as a general and promising target for cancer treatment. In this study, we examined the anti-tumor effects of CHM-1, a synthetic 6,7-methylenedioxy substituted 2-phenyl-4-quinolone derivative, on GBM cells in vitro and in vivo. CHM-1 selectively blocked IGF-1R auto-phosphorylation, with an ability to distinguish between IGF-1R and related tyrosine kinase receptors, such as insulin receptor (IR), epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), and fibroblast growth factor receptor (FGFR). Further investigation revealed that, the phosphorylation of ERK1/2 as well as Akt was inhibited in CHM-1 treated GBM8401 cells possibly through the selective blockage of IGF-1R auto-phosphorylation. Our study also showed that p.o. treatment with the hydrophilic dihydrogen phosphate CHM-1P reduced the tumor volumes of the GBM8401 derived tumors in mouse brain and also prolonged the survival rate. The results provided potential opportunities for effective chemotherapy for GBM.
CHM-1 Suppresses Formation of Cell Surface-associated GRP78-p85alpha Complexes, Inhibiting PI3K-AKT Signaling and Inducing Apoptosis of Human Nasopharyngeal Carcinoma Cells.[Pubmed:26408697]
Anticancer Res. 2015 Oct;35(10):5359-68.
The endoplasmic reticulum chaperone glucose-regulated protein 78 (GRP78) is selectively expressed on the surface of cancer cells, and contributes to the survival of cancer cells by forming complexes with p85alpha and promoting phosphatidylinositol 3-kinase-protein kinase B (PI3K-AKT) signaling. Hereiotain we report that 2'-fluoro-6,7-methylenedioxy-2-phenyl-4-quinolone (CHM-1) induces apoptosis of human nasopharyngeal carcinoma (NPC) cells, as characterized by morphological changes, DNA fragmentation, caspase-3 activation, and cleavage of poly (ADP-ribose) polymerase. Using cell surface biotinylation, flow cytometric analysis, co-immunoprecipitation, and ectopic expression of GRP78, we demonstrated that the attenuation of the cell surface localization and complex formation with p85alpha of GRP78 by CHM-1 was involved in the inhibition of PI3K-AKT signaling and the induction of apoptosis. CHM-1 treatment induced phosphorylation on Thr 69 of B cell lymphoma 2 and inhibited phosphorylation of Ser 136 on Bcl-2-associated death promoter, that were reversed by overexpression of GRP78. We further observed that loss of mitochondrial membrane potential and increase in reactive oxygen species content, release of mitochondrial cytochrome c, caspase-9 activation, and apoptotic cell death induced by CHM-1, were suppressed by treatment with cyclosporine A, and by the overexpression of constitutively active AKT1 or GRP78. These results indicate that CHM-1 induces NPC cell apoptosis by suppressing the formation of the cell surface-associated GRP78-PI3K-AKT signaling complex, likely through inhibition of the formation of cell surface-associated GRP78-p85alpha complexes.
CHM-1, a new vascular targeting agent, induces apoptosis of human umbilical vein endothelial cells via p53-mediated death receptor 5 up-regulation.[Pubmed:20007968]
J Biol Chem. 2010 Feb 19;285(8):5497-506.
CHM-1 (2'-fluoro-6,7-methylenedioxy-2-phenyl-4-quinolone) has been identified as a potent antitumor agent in human hepatocellular carcinoma; however, its role in tumor angiogenesis is unclear. This study investigated the effects of CHM-1 and the mechanisms by which it exerts its antiangiogenic and vascular disrupting properties. Using a xenograft model antitumor assay, we found that CHM-1 significantly inhibits tumor growth and microvessel formation. Flow cytometry, immunofluorescence microscopy, and cell death enzyme-linked immunosorbent assay kit revealed that CHM-1 inhibits growth of human umbilical vein endothelial cells (HUVEC) by induction of apoptotic cell death in a concentration-dependent manner. CHM-1 also suppresses HUVEC migration and capillary-like tube formation. We were able to correlate CHM-1-induced apoptosis in HUVEC with the cleavage of procaspase-3, -7, and -8, as well as with the cleavage of poly(ADP-ribose) polymerase by Western blotting assay. Such sensitization was achieved through up-regulation of death receptor 5 (DR5) but not DR4 or Fas. CHM-1 was also capable of increasing the expression level of p53, and most importantly, the induction of DR5 by CHM-1 was abolished by p53 small interfering RNA. Taken together, the results of this study indicate that CHM-1 exhibits vascular targeting activity associated with the induction of DR5-mediated endothelial cell apoptosis through p53 up-regulation, which suggests its potential as an antivascular and antitumor therapeutic agent.
CHM-1, a novel synthetic quinolone with potent and selective antimitotic antitumor activity against human hepatocellular carcinoma in vitro and in vivo.[Pubmed:18281518]
Mol Cancer Ther. 2008 Feb;7(2):350-60.
Hepatocellular carcinoma is highly chemoresistant to currently available chemotherapeutic agents. In this study, 2'-fluoro-6,7-methylenedioxy-2-phenyl-4-quinolone (CHM-1), a synthetic 6,7-substituted 2-phenyl-4-quinolone, was identified as a potent and selective antitumor agent in human hepatocellular carcinoma. CHM-1 induced growth inhibition of HA22T, Hep3B, and HepG2 cells in a concentration-dependent manner but did not obviously impair the viability of normal cells at the IC(50) for liver cancer cells. CHM-1-induced apoptosis was also characterized by immunofluorescence microscopy. CHM-1 interacted with tubulin at the colchicine-binding site, markedly inhibited tubulin polymerization both in vitro and in vivo, and disrupted microtubule organization. CHM-1 caused cell cycle arrest at G(2)-M phase by activating Cdc2/cyclin B1 complex activity. CHM-1-induced cell death, activation of Cdc2 kinase activity, and elevation of MPM2 phosphoepitopes were profoundly attenuated by roscovitine, a specific cyclin-dependent kinase inhibitor. CHM-1 did not modulate the caspase cascade, and the pan-caspase-inhibitor z-VAD-fmk did not abolish CHM-1-induced cell death. However, CHM-1 induced the translocation of apoptosis-inducing factor (AIF) from mitochondria to the nucleus. Small interfering RNA targeting of AIF substantially attenuated CHM-1-induced AIF translocation. Importantly, CHM-1 inhibited tumor growth and prolonged the lifespan in mice inoculated with HA22T cells. In conclusion, we show that CHM-1 exhibits a novel antimitotic antitumor activity against human hepatocellular carcinoma both in vitro and in vivo via a caspase-independent pathway. CHM-1 is a promising chemotherapeutic agent worthy of further development into a clinical trial candidate for treating cancer, especially hepatocellular carcinoma.


