LysicamineCAS# 15444-20-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 15444-20-9 | SDF | Download SDF |
| PubChem ID | 122691 | Appearance | Powder |
| Formula | C18H13NO3 | M.Wt | 291.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
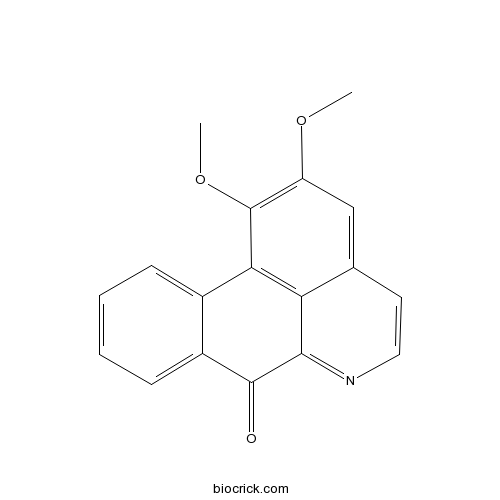
| SMILES | COC1=C(C2=C3C(=C1)C=CN=C3C(=O)C4=CC=CC=C42)OC | ||
| Standard InChIKey | DPBMWJXWUINLQT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H13NO3/c1-21-13-9-10-7-8-19-16-14(10)15(18(13)22-2)11-5-3-4-6-12(11)17(16)20/h3-9H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Lysicamine has antimicrobial and anti-inflammation activity, the minimum inhibitory concentrations of the pour component (Streptococcus mutans, Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans and Candida albicans.) is less than 5mg/ml for all four microorganisms and the minimum concentration with anti-inflammation is 0.2mg/ml for both IL-8 and IL-6. 2. Lysicamine has antileishmanial activity. 3. Lysicamine shows significant antioxidant capacity in the ORAC(FL) assay and it is active against S. epidermidis and C. dubliniensis, with MIC values in the range 12.5-100 microg mL(-1). 4. Lysicamine can significantly inhibit the proliferation of melanoma cells, it has antioxidative activity and chemopreventive activity in skin melanoma cells. |
| Targets | IL Receptor | Antifection |

Lysicamine Dilution Calculator

Lysicamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4329 mL | 17.1644 mL | 34.3289 mL | 68.6577 mL | 85.8222 mL |
| 5 mM | 0.6866 mL | 3.4329 mL | 6.8658 mL | 13.7315 mL | 17.1644 mL |
| 10 mM | 0.3433 mL | 1.7164 mL | 3.4329 mL | 6.8658 mL | 8.5822 mL |
| 50 mM | 0.0687 mL | 0.3433 mL | 0.6866 mL | 1.3732 mL | 1.7164 mL |
| 100 mM | 0.0343 mL | 0.1716 mL | 0.3433 mL | 0.6866 mL | 0.8582 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Zinc protoporphyrin IX
Catalog No.:BCC6775
CAS No.:15442-64-5
- 5,5'-Dimethoxylariciresinol 4-O-glucoside
Catalog No.:BCN1556
CAS No.:154418-16-3
- Methyl 3-O-feruloylquinate
Catalog No.:BCN3403
CAS No.:154418-15-2
- Capecitabine
Catalog No.:BCN2168
CAS No.:154361-50-9
- Echistatin, α1 isoform
Catalog No.:BCC5988
CAS No.:154303-05-6
- CB-5083
Catalog No.:BCC6528
CAS No.:1542705-92-9
- Ampalex
Catalog No.:BCC1359
CAS No.:154235-83-3
- Abiraterone
Catalog No.:BCC2259
CAS No.:154229-19-3
- Abiraterone Acotate
Catalog No.:BCN2184
CAS No.:154229-18-2
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- N-[2-Isopropylthiazol-4-ylmethyl(methyl)carbamoyl]-L-valine
Catalog No.:BCC9067
CAS No.:154212-61-0
- YM 90K hydrochloride
Catalog No.:BCC7455
CAS No.:154164-30-4
- Pramanicin
Catalog No.:BCN1853
CAS No.:154445-05-3
- Fmoc-Arg(Pbf)-OH
Catalog No.:BCC3040
CAS No.:154445-77-9
- NU 7026
Catalog No.:BCC3933
CAS No.:154447-35-5
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- LY 303511
Catalog No.:BCC1715
CAS No.:154447-38-8
- Methyl 5-O-feruloylquinate
Catalog No.:BCN3402
CAS No.:154461-64-0
- Sinapaldehyde glucoside
Catalog No.:BCN1689
CAS No.:154461-65-1
- CHM 1
Catalog No.:BCC2387
CAS No.:154554-41-3
- SB 204990
Catalog No.:BCC6342
CAS No.:154566-12-8
- Stauprimide
Catalog No.:BCC7768
CAS No.:154589-96-5
- Efavirenz
Catalog No.:BCC4135
CAS No.:154598-52-4
- Tezampanel
Catalog No.:BCC1993
CAS No.:154652-83-2
Antioxidant and anticancer constituents from the leaves of Liriodendron tulipifera.[Pubmed:24705566]
Molecules. 2014 Apr 3;19(4):4234-45.
Sixteen compounds were extracted and purified from the leaves of Liriodendron tulipifera. These compounds include aporphines, oxoaporphine, coumarin, sesquiterpene lactone, benzenoids, cyclitol and steroids. (+)-Norstephalagine (2) (an aporphine) and scopoletin (8) (a coumarin) were isolated from Liriodendron tulipifera leaves from the first time. The identified compounds were screened for their antiradical scavenging, metal chelating and ferric reducing power activities. The results have showed that these compounds have antioxidative activity. The study has also examined the chemopreventive property of the isolated compounds against human melanoma cells A375. The results shown that (-)-anonaine (1), (-)-liridinine (3), (+)-lirinidine (6), Lysicamine (7) and epitulipinolide diepoxide (9) significantly inhibited the proliferation of melanoma cells. These results revealed that these compounds have antioxidative activity and chemopreventive activity in skin melanoma cells.


