StauprimideInhibits NME2 nuclear translocation; primes ESCs for differentiation CAS# 154589-96-5 |
- Cucurbitacin I
Catalog No.:BCC2439
CAS No.:2222-07-3
- Niclosamide
Catalog No.:BCC5081
CAS No.:50-65-7
- NSC 74859
Catalog No.:BCC3701
CAS No.:501919-59-1
- WP1066
Catalog No.:BCC2194
CAS No.:857064-38-1
- SD 1008
Catalog No.:BCC2442
CAS No.:960201-81-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 154589-96-5 | SDF | Download SDF |
| PubChem ID | 46245328 | Appearance | Powder |
| Formula | C35H28N4O5 | M.Wt | 584.62 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
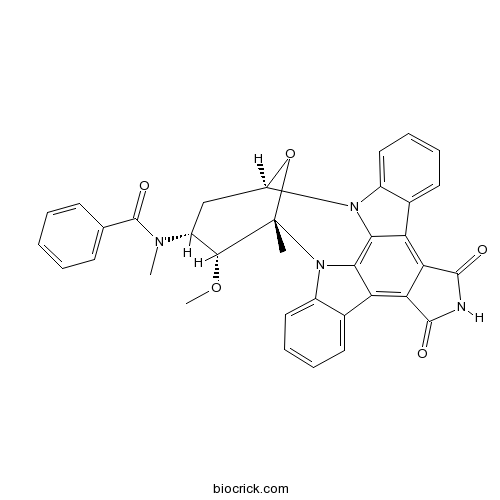
| Chemical Name | N-[(2S,3R,4R,6R)-3-methoxy-2-methyl-16,18-dioxo-29-oxa-1,7,17-triazaoctacyclo[12.12.2.12,6.07,28.08,13.015,19.020,27.021,26]nonacosa-8,10,12,14,19,21,23,25,27-nonaen-4-yl]-N-methylbenzamide | ||
| SMILES | CC12C(C(CC(O1)N3C4=CC=CC=C4C5=C6C(=C7C8=CC=CC=C8N2C7=C53)C(=O)NC6=O)N(C)C(=O)C9=CC=CC=C9)OC | ||
| Standard InChIKey | MQCCJEYZKWZQHU-MPRCCEKMSA-N | ||
| Standard InChI | InChI=1S/C35H28N4O5/c1-35-31(43-3)23(37(2)34(42)18-11-5-4-6-12-18)17-24(44-35)38-21-15-9-7-13-19(21)25-27-28(33(41)36-32(27)40)26-20-14-8-10-16-22(20)39(35)30(26)29(25)38/h4-16,23-24,31H,17H2,1-3H3,(H,36,40,41)/t23-,24-,31-,35+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Small molecule that primes embryonic stem cells (ESCs) for differentiation. Interacts with NME2 and inhibits its nuclear translocation. Increases the efficiency of directed differentiation of mouse and human ESCs in vitro. |

Stauprimide Dilution Calculator

Stauprimide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7105 mL | 8.5526 mL | 17.1051 mL | 34.2103 mL | 42.7628 mL |
| 5 mM | 0.3421 mL | 1.7105 mL | 3.421 mL | 6.8421 mL | 8.5526 mL |
| 10 mM | 0.1711 mL | 0.8553 mL | 1.7105 mL | 3.421 mL | 4.2763 mL |
| 50 mM | 0.0342 mL | 0.1711 mL | 0.3421 mL | 0.6842 mL | 0.8553 mL |
| 100 mM | 0.0171 mL | 0.0855 mL | 0.1711 mL | 0.3421 mL | 0.4276 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- SB 204990
Catalog No.:BCC6342
CAS No.:154566-12-8
- CHM 1
Catalog No.:BCC2387
CAS No.:154554-41-3
- Sinapaldehyde glucoside
Catalog No.:BCN1689
CAS No.:154461-65-1
- Methyl 5-O-feruloylquinate
Catalog No.:BCN3402
CAS No.:154461-64-0
- LY 303511
Catalog No.:BCC1715
CAS No.:154447-38-8
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- NU 7026
Catalog No.:BCC3933
CAS No.:154447-35-5
- Fmoc-Arg(Pbf)-OH
Catalog No.:BCC3040
CAS No.:154445-77-9
- Pramanicin
Catalog No.:BCN1853
CAS No.:154445-05-3
- Lysicamine
Catalog No.:BCN6523
CAS No.:15444-20-9
- Zinc protoporphyrin IX
Catalog No.:BCC6775
CAS No.:15442-64-5
- 5,5'-Dimethoxylariciresinol 4-O-glucoside
Catalog No.:BCN1556
CAS No.:154418-16-3
- Efavirenz
Catalog No.:BCC4135
CAS No.:154598-52-4
- Tezampanel
Catalog No.:BCC1993
CAS No.:154652-83-2
- 3-Methoxymollugin
Catalog No.:BCN7164
CAS No.:154706-44-2
- 2-(2'-Hydroxytetracosanoylamino)-octadecane-1,3,4-triol
Catalog No.:BCN1555
CAS No.:154801-30-6
- Cimiside E
Catalog No.:BCN7951
CAS No.:154822-57-8
- 4-Acetoxycinnamic acid
Catalog No.:BCN5026
CAS No.:15486-19-8
- Eleutheroside C
Catalog No.:BCN1690
CAS No.:15486-24-5
- 3,5-Dihydroxy-4',7-dimethoxyflavone
Catalog No.:BCN1691
CAS No.:15486-33-6
- Kaempferol 3,7,4'-trimethylether
Catalog No.:BCN4087
CAS No.:15486-34-7
- LL 37
Catalog No.:BCC8027
CAS No.:154947-66-7
- BAY-u 9773
Catalog No.:BCC7576
CAS No.:154978-38-8
- Erysenegalensein E
Catalog No.:BCN3979
CAS No.:154992-17-3
Stauprimide Priming of Human Embryonic Stem Cells toward Definitive Endoderm.[Pubmed:24518969]
Cell J. 2014 Feb 3;16(1):63-72. Epub 2014 Feb 3.
OBJECTIVE: In vitro production of a definitive endoderm (DE) is an important issue in stem cell-related differentiation studies and it can assist with the production of more efficient endoderm derivatives for therapeutic applications. Despite tremendous progress in DE differentiation of human embryonic stem cells (hESCs), researchers have yet to discover universal, efficient and cost-effective protocols. MATERIALS AND METHODS: In this experimental study, we have treated hESCs with 200 nM of Stauprimide (Spd) for one day followed by activin A (50 ng/ml; A50) for the next three days (Spd-A50). In the positive control group, hESCs were treated with Wnt3a (25 ng/ml) and activin A (100 ng/ml) for the first day followed by activin A for the next three days (100 ng/ml; W/A100-A100). RESULTS: Gene expression analysis showed up regulation of DE-specific marker genes (SOX17, FOXA2 and CXCR4) comparable to that observed in the positive control group. Expression of the other lineage specific markers did not significantly change (p<0.05). We also obtained the same gene expression results using another hESC line. The use of higher concentrations of Spd (400 and 800 nM) in the Spd-A50 protocol caused an increase in the expression SOX17 as well as a dramatic increase in mortality rate of the hESCs. A lower concentration of activin A (25 ng/ml) was not able to up regulate the DE-specific marker genes. Then, A50 was replaced by inducers of definitive endoderm; IDE1/2 (IDE1 and IDE2), two previously reported small molecule (SM) inducers of DE, in our protocol (Spd-IDE1/2). This replacement resulted in the up regulation of visceral endoderm (VE) marker (SOX7) but not DE-specific markers. Therefore, while the Spd-A50 protocol led to DE production, we have shown that IDE1/2 could not fully replace activin A in DE induction of hESCs. CONCLUSION: These findings can assist with the design of more efficient chemically-defined protocols for DE induction of hESCs and lead to a better understanding of the different signaling networks that are involved in DE differentiation of hESCs.
A small molecule primes embryonic stem cells for differentiation.[Pubmed:19427291]
Cell Stem Cell. 2009 May 8;4(5):416-26.
Embryonic stem cells (ESCs) are an attractive source of cells for disease modeling in vitro and may eventually provide access to cells/tissues for the treatment of many degenerative diseases. However, applications of ESC-derived cell types are largely hindered by the lack of highly efficient methods for lineage-specific differentiation. Using a high-content screen, we have identified a small molecule, named Stauprimide, that increases the efficiency of the directed differentiation of mouse and human ESCs in synergy with defined extracellular signaling cues. Affinity-based methods revealed that Stauprimide interacts with NME2 and inhibits its nuclear localization. This, in turn, leads to downregulation of c-Myc, a key regulator of the pluripotent state. Thus, our findings identify a chemical tool that primes ESCs for efficient differentiation through a mechanism that affects c-Myc expression, and this study points to an important role for NME2 in ESC self-renewal.


