NiclosamideSTAT3 and mTORC1 signaling inhibitor; antineoplastic against AML stem cells CAS# 50-65-7 |
- Sulfo-NHS-Biotin
Catalog No.:BCC3576
CAS No.:119616-38-5
- Sulfo-NHS-LC-Biotin
Catalog No.:BCC3578
CAS No.:127062-22-0
- NHS-Biotin
Catalog No.:BCC3577
CAS No.:35013-72-0
- Biotin Hydrazide
Catalog No.:BCC3582
CAS No.:66640-86-6
- NHS-LC-Biotin
Catalog No.:BCC3579
CAS No.:72040-63-2
- Iodoacetyl-LC-Biotin
Catalog No.:BCC3584
CAS No.:93285-75-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 50-65-7 | SDF | Download SDF |
| PubChem ID | 4477 | Appearance | Powder |
| Formula | C13H8Cl2N2O4 | M.Wt | 327.12 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BAY2353 | ||
| Solubility | DMF : 5 mg/mL (15.28 mM; Need ultrasonic) DMSO : 4.55 mg/mL (13.91 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
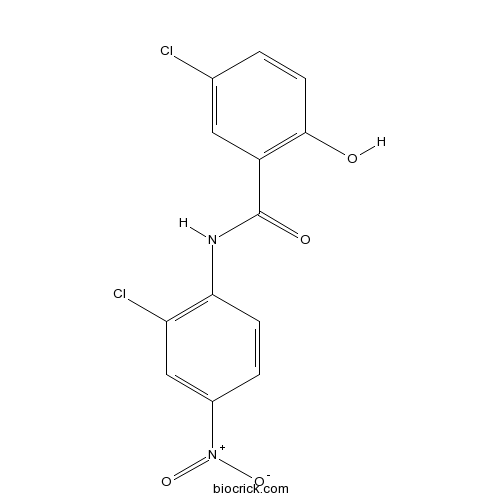
| Chemical Name | 5-chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxybenzamide | ||
| SMILES | C1=CC(=C(C=C1[N+](=O)[O-])Cl)NC(=O)C2=C(C=CC(=C2)Cl)O | ||
| Standard InChIKey | RJMUSRYZPJIFPJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H8Cl2N2O4/c14-7-1-4-12(18)9(5-7)13(19)16-11-3-2-8(17(20)21)6-10(11)15/h1-6,18H,(H,16,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of the STAT3 signaling pathway; inhibits the activation, nuclear translocation and transactivation of STAT3. Displays selectivity for STAT3 over STAT1, STAT5, JAK1, JAK2 and Src kinases. Inhibits the transcription of STAT3 target genes and induces cell growth inhibition, apoptosis and cell cycle arrest of cancer cells with constitutively active STAT3. Also reversibly inhibits mTORC1 signaling and stimulates autophagy in vitro; displays antineoplastic effects in acute myelogenous leukemia (AML) stem cells. | |||||

Niclosamide Dilution Calculator

Niclosamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.057 mL | 15.2849 mL | 30.5698 mL | 61.1396 mL | 76.4246 mL |
| 5 mM | 0.6114 mL | 3.057 mL | 6.114 mL | 12.2279 mL | 15.2849 mL |
| 10 mM | 0.3057 mL | 1.5285 mL | 3.057 mL | 6.114 mL | 7.6425 mL |
| 50 mM | 0.0611 mL | 0.3057 mL | 0.6114 mL | 1.2228 mL | 1.5285 mL |
| 100 mM | 0.0306 mL | 0.1528 mL | 0.3057 mL | 0.6114 mL | 0.7642 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Niclosamide can inhibit DNA replication and inhibit STAT with IC50 of 0.7 μM.
- Chloroquine diphosphate
Catalog No.:BCC3915
CAS No.:50-63-5
- Oxytocin
Catalog No.:BCC5435
CAS No.:50-56-6
- Reserpine
Catalog No.:BCN4960
CAS No.:50-55-5
- Estradiol Benzoate
Catalog No.:BCC4779
CAS No.:50-50-0
- Mercaptopurine (6-MP)
Catalog No.:BCC1186
CAS No.:50-44-2
- Clomiphene citrate
Catalog No.:BCC4480
CAS No.:50-41-9
- Cocaine
Catalog No.:BCN1901
CAS No.:50-36-2
- Thalidomide
Catalog No.:BCC2248
CAS No.:50-35-1
- Phenylbutazone
Catalog No.:BCC4822
CAS No.:50-33-9
- beta-Estradiol
Catalog No.:BCN2194
CAS No.:50-28-2
- Estriol
Catalog No.:BCN2235
CAS No.:50-27-1
- Prednisolone
Catalog No.:BCC4830
CAS No.:50-24-8
- 5-Hydroxytryptamine
Catalog No.:BCC9204
CAS No.:50-67-9
- 1,3:2,4-Di-p-methylbenyliedene sorbitol
Catalog No.:BCC4847
CAS No.:54686-97-4
- Actinomycin D
Catalog No.:BCC2385
CAS No.:50-76-0
- Aspirin (Acetylsalicylic acid)
Catalog No.:BCC2097
CAS No.:50-78-2
- Ascorbic acid
Catalog No.:BCN2207
CAS No.:50-81-7
- Thymidine
Catalog No.:BCN5622
CAS No.:50-89-5
- Floxuridine
Catalog No.:BCC1187
CAS No.:50-91-9
- Ephedrine Hydrochloride
Catalog No.:BCC8322
CAS No.:50-98-6
- D-(+)-Glucose
Catalog No.:BCN1259
CAS No.:50-99-7
- Nordihydroguaiaretic acid
Catalog No.:BCC1805
CAS No.:500-38-9
- L-Mimosine
Catalog No.:BCC5450
CAS No.:500-44-7
- Apoatropine
Catalog No.:BCN1869
CAS No.:500-55-0
Drug Repurposing of the Anthelmintic Niclosamide to Treat Multidrug-Resistant Leukemia.[Pubmed:28344555]
Front Pharmacol. 2017 Mar 10;8:110.
Multidrug resistance, a major problem that leads to failure of anticancer chemotherapy, requires the development of new drugs. Repurposing of established drugs is a promising approach for overcoming this problem. An example of such drugs is Niclosamide, a known anthelmintic that is now known to be cytotoxic and cytostatic against cancer cells. In this study, Niclosamide showed varying activity against different cancer cell lines. It revealed better activity against hematological cancer cell lines CCRF-CEM, CEM/ADR5000, and RPMI-8226 compared to the solid tumor cell lines MDA-MB-231, A549, and HT-29. The multidrug resistant CEM/ADR5000 cells were similar sensitive as their sensitive counterpart CCRF-CEM (resistance ration: 1.24). Furthermore, Niclosamide caused elevations in reactive oxygen species and glutathione (GSH) levels in leukemia cells. GSH synthetase (GS) was predicted as a target of Niclosamide. Molecular docking showed that Niclosamide probably binds to the ATP-binding site of GS with a binding energy of -9.40 kcal/mol. Using microscale thermophoresis, the binding affinity between Niclosamide and recombinant human GS was measured (binding constant: 5.64 muM). COMPARE analyses of the NCI microarray database for 60 cell lines showed that several genes, including those involved in lipid metabolism, correlated with cellular responsiveness to Niclosamide. Hierarchical cluster analysis showed five major branches with significant differences between sensitive and resistant cell lines (p = 8.66 x 10(5)). Niclosamide significantly decreased nuclear factor of activated T-cells (NFAT) activity as predicted by promoter binding motif analysis. In conclusion, Niclosamide was more active against hematological malignancies compared to solid tumors. The drug was particularly active against the multidrug-resistant CEM/ADR5000 leukemia cells. Inhibition of GSH synthesis and NFAT signaling were identified as relevant mechanisms for the anticancer activity of Niclosamide. Gene expression profiling predicted the sensitivity or resistance of cancer cells to Niclosamide.
The antihelmenthic phosphate niclosamide impedes renal fibrosis by inhibiting homeodomain-interacting protein kinase 2 expression.[Pubmed:28318631]
Kidney Int. 2017 Sep;92(3):612-624.
Renal fibrosis is the final common pathway of all varieties of progressive chronic kidney disease. However, there are no effective therapies to prevent or slow the progression of renal fibrosis. Niclosamide is a US Food and Drug Administration-approved oral antihelminthic drug used for treating most tapeworm infections. Here, we demonstrated that phosphate Niclosamide, the water-soluble form of Niclosamide, significantly reduced proteinuria, glomerulosclrotic lesions, and interstitial fibrosis in a murine model of adriamycin nephropathy. In addition, phosphate Niclosamide significantly ameliorated established renal interstitial fibrosis a murine model of unilateral ureteral obstruction. Mechanistically, phosphate Niclosamide directly inhibited TGF-beta-induced expression of homeodomain-interacting protein kinase 2 (HIPK2) by interfering with the binding of Smad3 to the promoter of the HIPK2 gene, and subsequently mitigated the activation of its downstream signaling pathways including Smad, Notch, NF-kappaB and Wnt/beta-catenin pathway both in vitro and in vivo. Thus, phosphate Niclosamide mitigates renal fibrosis at least partially by inhibiting HIPK2 expression. Hence, phosphate Niclosamide might be a potential therapeutic agent for renal fibrosis.
Dissipation, residues and risk assessment of metaldehyde and niclosamide ethanolamine in pakchoi after field application.[Pubmed:28372221]
Food Chem. 2017 Aug 15;229:604-609.
A method using LC-MS/MS after QuEChERS preparation for the simultaneous determination of metaldehyde and Niclosamide ethanolamine residues in soil and pakchoi has been developed and validated. The mean recoveries were ranged from 90% to 101% with RSDs (relative standard deviations) less than 9.2%. The dissipation results showed that the half-lives of metaldehyde and Niclosamide ethanolamine were 2.3-4.3d and 1.7-9.5d, respectively. The terminal residue results indicated that the residues of metaldehyde in pakchoi were lower than the temporary maximum residue limits (MRL) set by China on 1 d after last treatment and the maximum residue of Niclosamide ethanolamine in pakchoi was 0.54mg/kg. The risk quotients of metaldehyde and Niclosamide ethanolamine were ranged from 0.015 to 0.033 and from 0.00064 to 0.0014, respectively. This work could provide guidance on reasonable use of these molluscicides and aid in the establishment of MRL in China.
Niclosamide inhibition of STAT3 synergizes with erlotinib in human colon cancer.[Pubmed:28367059]
Onco Targets Ther. 2017 Mar 23;10:1767-1776.
Niclosamide, an anthelmintic drug approved by the US Food and Drug Administration against cestodes, is used to treat tapeworm infection. In this study, we show that Niclosamide can potentially inhibit signal transducer and activator of transcription 3 (STAT3) in colon cancer cell lines. Combined inhibition of epidermal growth factor receptor and STAT3 by erlotinib and Niclosamide synergistically induces apoptosis and antiproliferation in colon cancer cell lines. Our findings suggest that erlotinib and Niclosamide combination provides an effective therapeutic approach to improving the prognosis of colon cancer.
Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: inactivation of the NF-kappaB pathway and generation of reactive oxygen species.[Pubmed:20215516]
Cancer Res. 2010 Mar 15;70(6):2516-27.
NF-kappaB may be a potential therapeutic target for acute myelogenous leukemia (AML) because NF-kappaB activation is found in primitive human AML blast cells. In this report, we initially discovered that the potent antineoplastic effect of Niclosamide, a Food and Drug Administration-approved antihelminthic agent, was through inhibition of the NF-kappaB pathway in AML cells. Niclosamide inhibited the transcription and DNA binding of NF-kappaB. It blocked tumor necrosis factor-induced IkappaBalpha phosphorylation, translocation of p65, and expression of NF-kappaB-regulated genes. Niclosamide inhibited the steps TAK1-->IkappaB kinase (IKK) and IKK-->IkappaBalpha. Niclosamide also increased the levels of reactive oxygen species (ROS) in AML cells. Quenching ROS by the glutathione precursor N-acetylcysteine attenuated Niclosamide-induced apoptosis. Our results together suggest that Niclosamide inhibited the NF-kappaB pathway and increased ROS levels to induce apoptosis in AML cells. On translational study of the efficacy of Niclosamide against AML, Niclosamide killed progenitor/stem cells from AML patients but spared those from normal bone marrow. Niclosamide was synergistic with the frontline chemotherapeutic agents cytarabine, etoposide, and daunorubicin. It potently inhibited the growth of AML cells in vitro and in nude mice. Our results support further investigation of Niclosamide in clinical trials of AML patients.
Screen for chemical modulators of autophagy reveals novel therapeutic inhibitors of mTORC1 signaling.[Pubmed:19771169]
PLoS One. 2009 Sep 22;4(9):e7124.
BACKGROUND: Mammalian target of rapamycin complex 1 (mTORC1) is a protein kinase that relays nutrient availability signals to control numerous cellular functions including autophagy, a process of cellular self-eating activated by nutrient depletion. Addressing the therapeutic potential of modulating mTORC1 signaling and autophagy in human disease requires active chemicals with pharmacologically desirable properties. METHODOLOGY/PRINCIPAL FINDINGS: Using an automated cell-based assay, we screened a collection of >3,500 chemicals and identified three approved drugs (perhexiline, Niclosamide, amiodarone) and one pharmacological reagent (rottlerin) capable of rapidly increasing autophagosome content. Biochemical assays showed that the four compounds stimulate autophagy and inhibit mTORC1 signaling in cells maintained in nutrient-rich conditions. The compounds did not inhibit mTORC2, which also contains mTOR as a catalytic subunit, suggesting that they do not inhibit mTOR catalytic activity but rather inhibit signaling to mTORC1. mTORC1 inhibition and autophagosome accumulation induced by perhexiline, Niclosamide or rottlerin were rapidly reversed upon drug withdrawal whereas amiodarone inhibited mTORC1 essentially irreversibly. TSC2, a negative regulator of mTORC1, was required for inhibition of mTORC1 signaling by rottlerin but not for mTORC1 inhibition by perhexiline, Niclosamide and amiodarone. Transient exposure of immortalized mouse embryo fibroblasts to these drugs was not toxic in nutrient-rich conditions but led to rapid cell death by apoptosis in starvation conditions, by a mechanism determined in large part by the tuberous sclerosis complex protein TSC2, an upstream regulator of mTORC1. By contrast, transient exposure to the mTORC1 inhibitor rapamycin caused essentially irreversible mTORC1 inhibition, sustained inhibition of cell growth and no selective cell killing in starvation. CONCLUSION/SIGNIFICANCE: The observation that drugs already approved for human use can reversibly inhibit mTORC1 and stimulate autophagy should greatly facilitate the preclinical and clinical testing of mTORC1 inhibition for indications such as tuberous sclerosis, diabetes, cardiovascular disease and cancer.


