Actinomycin DRNA polymerase inhibitor CAS# 50-76-0 |
- VX-222 (VCH-222, Lomibuvir)
Catalog No.:BCC2108
CAS No.:1026785-59-0
- CHM 1
Catalog No.:BCC2387
CAS No.:154554-41-3
- T 705
Catalog No.:BCC4130
CAS No.:259793-96-9
- Gambogic acid
Catalog No.:BCN2318
CAS No.:2752-65-0
- Fidaxomicin
Catalog No.:BCC4660
CAS No.:873857-62-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 50-76-0 | SDF | Download SDF |
| PubChem ID | 2019 | Appearance | Powder |
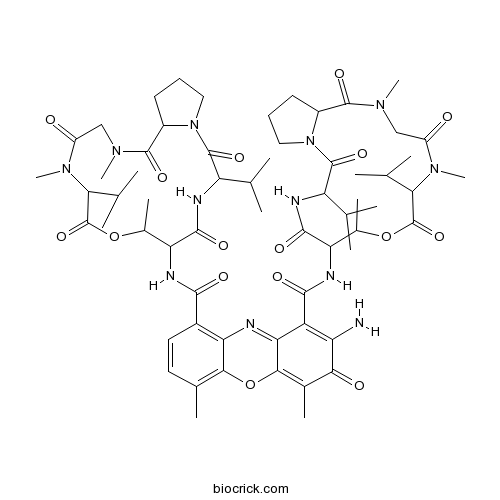
| Formula | C62H86N12O16 | M.Wt | 1255.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Dactinomycin; Actinomycin IV | ||
| Solubility | DMSO : ≥ 27 mg/mL (21.51 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-amino-4,6-dimethyl-3-oxo-1-N,9-N-bis[7,11,14-trimethyl-2,5,9,12,15-pentaoxo-3,10-di(propan-2-yl)-8-oxa-1,4,11,14-tetrazabicyclo[14.3.0]nonadecan-6-yl]phenoxazine-1,9-dicarboxamide | ||
| SMILES | CC1C(C(=O)NC(C(=O)N2CCCC2C(=O)N(CC(=O)N(C(C(=O)O1)C(C)C)C)C)C(C)C)NC(=O)C3=C4C(=C(C=C3)C)OC5=C(C(=O)C(=C(C5=N4)C(=O)NC6C(OC(=O)C(N(C(=O)CN(C(=O)C7CCCN7C(=O)C(NC6=O)C(C)C)C)C)C(C)C)C)N)C | ||
| Standard InChIKey | RJURFGZVJUQBHK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C62H86N12O16/c1-27(2)42-59(84)73-23-17-19-36(73)57(82)69(13)25-38(75)71(15)48(29(5)6)61(86)88-33(11)44(55(80)65-42)67-53(78)35-22-21-31(9)51-46(35)64-47-40(41(63)50(77)32(10)52(47)90-51)54(79)68-45-34(12)89-62(87)49(30(7)8)72(16)39(76)26-70(14)58(83)37-20-18-24-74(37)60(85)43(28(3)4)66-56(45)81/h21-22,27-30,33-34,36-37,42-45,48-49H,17-20,23-26,63H2,1-16H3,(H,65,80)(H,66,81)(H,67,78)(H,68,79) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Anti-neoplastic antibiotic. Inhibits RNA polymerase and is a potent inducer of apoptosis. |

Actinomycin D Dilution Calculator

Actinomycin D Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.7965 mL | 3.9827 mL | 7.9654 mL | 15.9308 mL | 19.9135 mL |
| 5 mM | 0.1593 mL | 0.7965 mL | 1.5931 mL | 3.1862 mL | 3.9827 mL |
| 10 mM | 0.0797 mL | 0.3983 mL | 0.7965 mL | 1.5931 mL | 1.9913 mL |
| 50 mM | 0.0159 mL | 0.0797 mL | 0.1593 mL | 0.3186 mL | 0.3983 mL |
| 100 mM | 0.008 mL | 0.0398 mL | 0.0797 mL | 0.1593 mL | 0.1991 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: Actinomycin D showed a concentration-dependent decrease of DNA repair activity with the IC50 of 0.42 μM [1].
Actinomycin D (dactinomycin), a member of actinomycines, which are a class of polypeptide antibiotics isolated from soil bacteria of the genus Streptomyces. Have been used for many years as an older chemotherapy, actinomycin D binds to double- and single-stranded DNA to inhibit DNA and RNA synthesis by binding DNA at the transcription initiation complex and preventing elongation of RNA chain by RNA polymerase.
In vitro: A previous study was designed to determine the effects of actinomycin D on leptin release by isolated rat adipocytes during primary culture for 24 hr. Results showed that both actinomycin D and dexamethasone reduced the loss of leptin mRNA seen over the 24-hr incubation. Maximal effects on leptin release and leptin mRNA accumulation required only 0.1 μM of actinomycin D, a concentration that had no significant effect on the 18S RNA content of adipocytes at the end of a 24-hr incubation. In contrast to the reduced loss of leptin mRNA seen at 24 hr, the loss of glyceraldehyde-3-phosphate dehydrogenase messenger ribonucleic acid (GAPDH mRNA) was enhanced in the presence of 0.1 μM of actinomycin D. These results demonstrated a unique regulation of leptin release and leptin mRNA levels by actinomycin D [2].
In vivo: A rat in vivo study showed that the effect of actinomycin D on the time course of the population spike potentiation was more pronounced than the effect on the time course of the EPSP component, suggesting different mechanisms for the two forms of potentiation. Moreover, both intrahippocampal and intracerebroventricular injection of actinomycin D prevented a late stage of LTP in the dentate gyrus in vivo measured as the population spike amplitude [3].
Clinical trial: Actinomycin is intravenously administered and most commonly used in the treatment of a variety of cancers, including gestational trophoblastic neoplasia, wilms' tumor, rhabdomyosarcoma, ewing's sarcoma and malignant hydatidiform mole. Combined with other drugs in chemotherapy regimens, such as the VAC regimen, it will be used for treating rhabdomyosarcoma and Ewing's Sarcoma. In addition, it is also used as a radiosensitizer in adjunct to radiotherapies, as it increases the tumor cells radiosensitivity.
Reference:
[1] Barret JM, Salles B, Provot C, Hill BT. Evaluation of DNA repair inhibition by antitumor or antibiotic drugs using a chemiluminescence microplate assay. Carcinogenesis. 1997 ;18(12):2441-5.
[2] Fain JN, Bahouth SW. Stimulation of leptin release by actinomycin D in rat adipocytes. Biochem Pharmacol. 1998;55(8):1309-14.
[3] Frey U, Frey S, Schollmeier F, Krug M. Influence of actinomycin D, a RNA synthesis inhibitor, on long-term potentiation in rat hippocampal neurons in vivo and in vitro. J Physiol. 1996;490(Pt 3):703-11.
- 1,3:2,4-Di-p-methylbenyliedene sorbitol
Catalog No.:BCC4847
CAS No.:54686-97-4
- 5-Hydroxytryptamine
Catalog No.:BCC9204
CAS No.:50-67-9
- Niclosamide
Catalog No.:BCC5081
CAS No.:50-65-7
- Chloroquine diphosphate
Catalog No.:BCC3915
CAS No.:50-63-5
- Oxytocin
Catalog No.:BCC5435
CAS No.:50-56-6
- Reserpine
Catalog No.:BCN4960
CAS No.:50-55-5
- Estradiol Benzoate
Catalog No.:BCC4779
CAS No.:50-50-0
- Mercaptopurine (6-MP)
Catalog No.:BCC1186
CAS No.:50-44-2
- Clomiphene citrate
Catalog No.:BCC4480
CAS No.:50-41-9
- Cocaine
Catalog No.:BCN1901
CAS No.:50-36-2
- Thalidomide
Catalog No.:BCC2248
CAS No.:50-35-1
- Phenylbutazone
Catalog No.:BCC4822
CAS No.:50-33-9
- Aspirin (Acetylsalicylic acid)
Catalog No.:BCC2097
CAS No.:50-78-2
- Ascorbic acid
Catalog No.:BCN2207
CAS No.:50-81-7
- Thymidine
Catalog No.:BCN5622
CAS No.:50-89-5
- Floxuridine
Catalog No.:BCC1187
CAS No.:50-91-9
- Ephedrine Hydrochloride
Catalog No.:BCC8322
CAS No.:50-98-6
- D-(+)-Glucose
Catalog No.:BCN1259
CAS No.:50-99-7
- Nordihydroguaiaretic acid
Catalog No.:BCC1805
CAS No.:500-38-9
- L-Mimosine
Catalog No.:BCC5450
CAS No.:500-44-7
- Apoatropine
Catalog No.:BCN1869
CAS No.:500-55-0
- Convolamine
Catalog No.:BCN1905
CAS No.:500-56-1
- Yangonin
Catalog No.:BCN3565
CAS No.:500-62-9
- Kawain
Catalog No.:BCN3564
CAS No.:500-64-1
Severe Hepatic Sinusoidal Obstruction Syndrome in a Child Receiving Vincristine, Actinomycin-D, and Cyclophosphamide for Rhabdomyosarcoma: Successful Treatment with Defibrotide.[Pubmed:27034141]
Cancer Res Treat. 2016 Oct;48(4):1443-1447.
Hepatic sinusoidal obstruction syndrome (SOS) is a life-threatening syndrome that generally occurs as a complication after hematopoietic stem cell transplantation or, less commonly, after conventional chemotherapy. Regarding SOS in rhabdomyosarcoma patients who received conventional chemotherapy, the doses of chemotherapeutic agents are associated with the development of SOS. Several cases of SOS in rhabdomyosarcoma patients after receiving chemotherapy with escalated doses of cyclophosphamide have been reported. Here, we report on a 9-year-old female with rhabdomyosarcoma who developed severe SOS after receiving chemotherapy consisting of vincristine, actinomycin-D, and a moderate dose of cyclophosphamide. She was treated successfully with defibrotide without sequelae to the liver.
Comparing and evaluating the efficacy of methotrexate and actinomycin D as first-line single chemotherapy agents in low risk gestational trophoblastic disease.[Pubmed:27819410]
J Gynecol Oncol. 2017 Mar;28(2):e8.
OBJECTIVE: The aim of this study was to compare responses to single-agent chemotherapies and evaluate the predictive factors of resistance in low risk (LR) gestational trophoblastic disease (GTD). The chemotherapy agents included methotrexate (MTX) and Actinomycin D (ACT-D). METHODS: We conducted a retrospective study of 126 patients with GTD who were treated between 2000 and 2013. A total of 71 patients with LR GTD were treated with MTX (8-day regimen or weekly regimen, n=53) or ACT-D (bi-weekly pulsed regimen or 5-day regimen, n=18). The successful treatment group and the failed treatment group were compared and analyzed to identify prognostic factors. RESULTS: The complete response rates were 83.3% for ACT-D and 62.2% for MTX, with no statistically significant difference. There was no severe adverse effect reported for either group. Longer interval durations from the index pregnancy (>2 months, p=0.040) and larger tumor size (>3 cm, p=0.020) were more common in non-responders than in responders; these results were statistically significant. CONCLUSION: Based on our results, ACT-D may be a better option than MTX as a first-line single chemotherapy agent for LR GTD. The bi-weekly pulsed ACT-D regimen had minimal, or at least the same, toxicities compared with MTX. However, due to the lack of strong supporting evidence, it cannot be conclusively stated that this is the best single agent for first-line chemotherapy in LR GTD patients. Further larger controlled trials will be necessary to establish the best guidelines for GTD treatment.
Human p53 interacts with the elongating RNAPII complex and is required for the release of actinomycin D induced transcription blockage.[Pubmed:28102346]
Sci Rep. 2017 Jan 19;7:40960.
The p53 tumour suppressor regulates the transcription initiation of selected genes by binding to specific DNA sequences at their promoters. Here we report a novel role of p53 in transcription elongation in human cells. Our data demonstrate that upon transcription elongation blockage, p53 is associated with genes that have not been reported as its direct targets. p53 could be co-immunoprecipitated with active forms of DNA-directed RNA polymerase II subunit 1 (RPB1), highlighting its association with the elongating RNA polymerase II. During a normal transcription cycle, p53 and RPB1 are localised at distinct regions of selected non-canonical p53 target genes and this pattern of localisation was changed upon blockage of transcription elongation. Additionally, transcription elongation blockage induced the proteasomal degradation of RPB1. Our results reveal a novel role of p53 in human cells during transcription elongation blockage that may facilitate the removal of RNA polymerase II from DNA.
The mechanism of actinomycin D-mediated inhibition of HIV-1 reverse transcription.[Pubmed:9826774]
Nucleic Acids Res. 1998 Dec 1;26(23):5472-9.
The mechanism of reverse transcription was analyzed in vitro with RNA templates and the reverse transcriptase (RT) enzyme of human immunodeficiency virus type 1 (HIV-1). In particular, we analyzed the mechanism of Actinomycin D (ActD) mediated inhibition of the strand transfer step, in which the newly synthesized cDNA, termed the (-) strand strong stop or (-)ssDNA, is transferred from the donor RNA onto the acceptor RNA. This strand transfer reaction is a rather inefficient process in vitro. We found that this is in part due to the presence of an excess donor RNA, and highly efficient strand transfer was achieved by reducing the amount of donor RNA. We suggest that annealing of the (-)ssDNA to the excess donor RNA is preferred over productive binding to the acceptor RNA because of a higher basepair complementarity. ActD remains a potent inhibitor of strand transfer in this optimized assay system. We measured no effect of ActD on the elongation of reverse transcription or the RNase H action of the RT enzyme. Instead, we provide evidence that ActD acts through direct interaction with the (-)ssDNA, thereby blocking the basepairing capacity of this molecule. The possible use of single-stranded DNA binding molecules as antiretroviral agents is discussed.
A kinetic study on the mechanism of inhibition of RNA synthesis catalyzed by DNA-dependent RNA polymerase. Differences in inhibition by ethidium bromide, 3,8-diamino-6-ethylphenanthridinium bromide and actinomycin d.[Pubmed:7025910]
Biochim Biophys Acta. 1981 Oct 27;655(3):278-90.
The mechanism of inhibition of RNA polymerase-catalyzed synthesis of RNA by Actinomycin D and the phenan-thridinium derivatives ethidium bromide and 3,8-diamino-6-ethylphenanthridinium bromide (DEMB) is examined. A general kinetic equation describing the dependence of RNA synthesis on DNA template concentration is derived and distinct expressions corresponding to various possible mechanisms of inhibition are subsequently obtained by introducing into the equations assumptions as appropriate for the individual mechanisms. The fitting of the experimental results of inhibition into the resulting equations suggested that the ethidium bromide and DEMB inhibit RNA polymerase by forming an inhibitor-template complex which interferes with enzyme recognition of, and binding to, appropriate sites on the template (binding inhibition). The fitting of the dependence of the rate of RNA synthesis on the bound-inhibitor to DNA ratios to the derived kinetic expressions also allows a tentative distinction to be made as to whether ethidium bromide and DEMB interfere with RNA synthesis by a mechanism of 'partial' or 'complete' inhibition.


