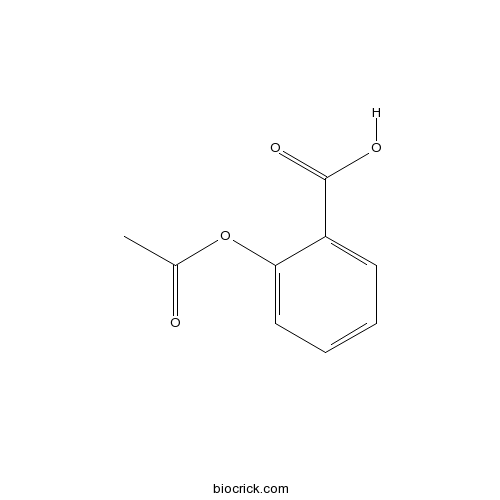
Aspirin (Acetylsalicylic acid)Cyclooxygenase (COX) inhibitor CAS# 50-78-2 |
- AP26113
Catalog No.:BCC1069
CAS No.:1197958-12-5
- LDK378 dihydrochloride
Catalog No.:BCC1694
CAS No.:1380575-43-8
- ALK inhibitor 1
Catalog No.:BCC1339
CAS No.:761436-81-1
- ALK inhibitor 2
Catalog No.:BCC1340
CAS No.:761438-38-4
- (R)-Crizotinib
Catalog No.:BCC1284
CAS No.:877399-52-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 50-78-2 | SDF | Download SDF |
| PubChem ID | 2244 | Appearance | Powder |
| Formula | C9H8O4 | M.Wt | 180.16 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ASA; Acetylsalicylic Acid | ||
| Solubility | DMSO : 100 mg/mL (555.06 mM; Need ultrasonic) H2O : 0.1 mg/mL (0.56 mM; Need ultrasonic) | ||
| Chemical Name | 2-acetyloxybenzoic acid | ||
| SMILES | CC(=O)OC1=CC=CC=C1C(=O)O | ||
| Standard InChIKey | BSYNRYMUTXBXSQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h2-5H,1H3,(H,11,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of cyclooxygenase (COX)-1. Blocks the production of prostaglandins and thromboxanes. Exhibits antiplatelet and antithrombotic activities. Displays anticancer effects in some solid tumors. One of the first described NSAID drugs. |

Aspirin (Acetylsalicylic acid) Dilution Calculator

Aspirin (Acetylsalicylic acid) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.5506 mL | 27.7531 mL | 55.5062 mL | 111.0124 mL | 138.7655 mL |
| 5 mM | 1.1101 mL | 5.5506 mL | 11.1012 mL | 22.2025 mL | 27.7531 mL |
| 10 mM | 0.5551 mL | 2.7753 mL | 5.5506 mL | 11.1012 mL | 13.8766 mL |
| 50 mM | 0.111 mL | 0.5551 mL | 1.1101 mL | 2.2202 mL | 2.7753 mL |
| 100 mM | 0.0555 mL | 0.2775 mL | 0.5551 mL | 1.1101 mL | 1.3877 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Aspirin (Acetylsalicylic acid) is a potent and selective inhibitor of cyclooxygenase (COX) with a broad range of pharmacological activities including anti-inflammation and pain relief. Multiple studies have accumulated sufficient evidence to establish the association between the use of aspirin and a reduced risk of cancers including prostate cancer, breast cancer, colorectal cancer, endometrial cancer, and ovarian cancer. Aspirin suppresses ovarian cancer cells harboring COX-1 by acting as histone deacetylase inhibitors to up-regulate cell cycle arrest protein p21. Aspirin also inhibits the expression of COX-2 in human umbilical vein endothelial cells and neonatal rat ventricular cardiomyocytes resulting in reduced PG production and the down-regulation of ERK and NF-KB respectively.
Reference
Cho M, Kabir SM, Dong Y, Lee E, Rice VM, Khabele D, Son DS. Aspirin Blocks EGF-stimulated Cell Viability in a COX-1 Dependent Manner in Ovarian Cancer Cells. J Cancer. 2013;4(8):671-678.
Duan Y, Chen F, Zhang A, Zhu B, Sun J, Xie Q, Chen Z. Aspirin inhibits lipopolysaccharide-induced COX-2 expression and PGE2 production in porcine alveolar macrophages by modulating protein kinase C and protein tyrosine phosphatase activity. BMB Rep. 2013. pii: 2320. [Epub ahead of print]
- Actinomycin D
Catalog No.:BCC2385
CAS No.:50-76-0
- 1,3:2,4-Di-p-methylbenyliedene sorbitol
Catalog No.:BCC4847
CAS No.:54686-97-4
- 5-Hydroxytryptamine
Catalog No.:BCC9204
CAS No.:50-67-9
- Niclosamide
Catalog No.:BCC5081
CAS No.:50-65-7
- Chloroquine diphosphate
Catalog No.:BCC3915
CAS No.:50-63-5
- Oxytocin
Catalog No.:BCC5435
CAS No.:50-56-6
- Reserpine
Catalog No.:BCN4960
CAS No.:50-55-5
- Estradiol Benzoate
Catalog No.:BCC4779
CAS No.:50-50-0
- Mercaptopurine (6-MP)
Catalog No.:BCC1186
CAS No.:50-44-2
- Clomiphene citrate
Catalog No.:BCC4480
CAS No.:50-41-9
- Cocaine
Catalog No.:BCN1901
CAS No.:50-36-2
- Thalidomide
Catalog No.:BCC2248
CAS No.:50-35-1
- Ascorbic acid
Catalog No.:BCN2207
CAS No.:50-81-7
- Thymidine
Catalog No.:BCN5622
CAS No.:50-89-5
- Floxuridine
Catalog No.:BCC1187
CAS No.:50-91-9
- Ephedrine Hydrochloride
Catalog No.:BCC8322
CAS No.:50-98-6
- D-(+)-Glucose
Catalog No.:BCN1259
CAS No.:50-99-7
- Nordihydroguaiaretic acid
Catalog No.:BCC1805
CAS No.:500-38-9
- L-Mimosine
Catalog No.:BCC5450
CAS No.:500-44-7
- Apoatropine
Catalog No.:BCN1869
CAS No.:500-55-0
- Convolamine
Catalog No.:BCN1905
CAS No.:500-56-1
- Yangonin
Catalog No.:BCN3565
CAS No.:500-62-9
- Kawain
Catalog No.:BCN3564
CAS No.:500-64-1
- Rhapontigenin
Catalog No.:BCN3515
CAS No.:500-65-2
Efficacy of acetylsalicylic acid (aspirin) in skin B16-F0 melanoma tumor-bearing C57BL/6 mice.[Pubmed:24492939]
Tumour Biol. 2014 May;35(5):4967-76.
Several epidemiological studies show that aspirin can act as a chemopreventive agent and decrease the incidences of various cancers including melanoma. In this work, we investigated the in vitro and in vivo efficacy of acetylsalicylic acid (ASA) as an antimelanoma agent in B16-F0 cells and skin B16-F0 melanoma tumor mouse model. Our findings indicate that the IC50 (48 h) for ASA in B16-F0 melanoma cells was 100 muM and that ASA caused a dose- and time-dependent GSH depletion and increase in reactive oxygen species (ROS) formation in B16-F0 melanoma cells. Male C57BL/6 mice were inoculated s.c. with 1 x 10(6) B16-F0 melanoma cells. ASA (80, 100, and 150 mg/kg) was initiated on day 1 or day 7, or day 9 after cell inoculation and continued daily for 13, 7, and 5 days, respectively. Animals were weighed daily and sacrificed on day 13. The tumors were excised and weighed. The animals receiving 13 days of ASA therapy at 80, 100, and 150 mg/kg demonstrated tumor growth inhibition by 1 +/- 12%, 19 +/- 22%, and 50 +/- 29%, respectively. Animals receiving 7 days of therapy at 80, 100, and 150 mg/kg demonstrated tumor growth inhibition by 12 +/- 14%, 27 +/- 14%, and 40 +/- 14%, respectively. No significant tumor growth inhibition was observed with 5 days of therapy. ASA at 100 and 150 mg/kg caused significant tumor growth inhibition in C57BL/6 mice when administered for 13 and 7 days, respectively. The results obtained in this study are consistent with the recent epidemiologically based report that aspirin is associated with lower melanoma risk in humans.
Epithelial MUC1 promotes cell migration, reduces apoptosis and affects levels of mucosal modulators during acetylsalicylic acid (aspirin)-induced gastropathy.[Pubmed:25387004]
Biochem J. 2015 Feb 1;465(3):423-31.
MUC1 is a transmembrane mucin highly expressed in the stomach. Although extensive research has uncovered many of its roles in cancer, knowledge about the functions of MUC1 in normal tissues is limited. In the present study, we showed that acetylsalicylic acid (ASA; aspirin) up-regulated MUC1/Muc1 expression in the gastric mucosa of humans and wild-type (WT) mice. ASA induced mucosal injury in all mice to a similar extent; however, WT animals and those chimaeras with Muc1 on the epithelia recovered faster than Muc1-knockout (KO) mice and chimaeras carrying Muc1 on haemopoietic but not epithelial cells. MUC1 enhanced proliferation and migration of the human gastric cell line MKN-7 and increased resistance to apoptosis. The repeated treatment regime used caused a reduction in cyclo-oxygenase-1 (Cox-1) expression, though WT animals returned faster towards pre-treatment levels and had increased Cox-2 and vascular endothelial growth factor levels during recovery. Thus we found that epithelial Muc1 is more important for the healing process than haemopoietic Muc1 and Muc1/MUC1 facilitates wound healing by enhancing cell migration and proliferation, protecting against apoptosis and mediating expression of mucosal modulators. Thus MUC1 plays essential roles during wound healing and development of treatment modalities targeting enhanced expression of MUC1 may be beneficial to treat mucosal wounds.
The effects of aspirin, flurbiprofen, and NO-donating acetylsalicylic acid (NCX 4016) on mice models of endotoxic and septic shock.[Pubmed:26422851]
Turk J Med Sci. 2015;45(4):812-9.
BACKGROUND/AIM: Nitric oxide-donating nonsteroidal antiinflammatory drugs (NO-NSAIDs) are a promising new class of antiinflammatory agents, which are obtained by adding NO-donating moieties to the existing conventional NSAID molecules. The aim of this study was to investigate the effects of aspirin, flurbiprofen, and NO-donating acetylsalicylic acid (NCX 4016) on cecal ligation and puncture (CLP) and endotoxin-induced septic shock (LPS) models in mice. MATERIALS AND METHODS: Overall survival and spleen and liver weights were monitored in LPS and CLP models. Histopathological examinations of liver and spleen were performed at the end of the experimental protocols. RESULTS: NCX 4016 was able to reverse the increased spleen weight in CLP-operated animals, whereas aspirin or flurbiprofen did not. Similar to the results of the CLP model, none of the drugs modified the survival rates in the LPS model. Flurbiprofen in particular produced significant histopathological damage in spleens and livers, which was less significant with aspirin. NCX 4016 did not cause any liver damage. CONCLUSION: NCX 4016 has the potential to be used in septic states, while special attention has to be paid to the effects of aspirin and flurbiprofen on the liver and spleen.
Acetylsalicylic acid (aspirin) and salicylic acid interaction with the human erythrocyte membrane bilayer induce in vitro changes in the morphology of erythrocytes.[Pubmed:24055635]
Arch Biochem Biophys. 2013 Nov 1;539(1):9-19.
Despite the well-documented information, there are insufficient reports concerning the effects of salicylate compounds on the structure and functions of cell membranes, particularly those of human erythrocytes. With the aim to better understand the molecular mechanisms of the interaction of acetylsalicylic acid (ASA) and salicylic acid (SA) with cell membranes, human erythrocyte membranes and molecular models were utilized. These consisted of bilayers of dimyristoylphosphatidylcholine (DMPC) and dimyristoylphosphatidylethanolamine (DMPE), representative of phospholipid classes located in the outer and inner monolayers of the human erythrocyte membrane, respectively. The capacity of ASA and SA to perturb the multibilayer structures of DMPC and DMPE was evaluated by X-ray diffraction while DMPC unilamellar vesicles (LUV) were studied by fluorescence spectroscopy. Moreover, we took advantage of the capability of differential scanning calorimetry (DSC) to detect the changes in the thermotropic phase behavior of lipid bilayers resulting from ASA and SA interaction with PC and PE molecules. In an attempt to further elucidate their effects on cell membranes, the present work also examined their influence on the morphology of intact human erythrocytes by means of defocusing and scanning electron microscopy, while isolated unsealed human erythrocyte membranes (IUM) were studied by fluorescence spectroscopy. Results indicated that both salicylates interact with human erythrocytes and their molecular models in a concentration-dependent manner perturbing their bilayer structures.
Aspirin and platelets: the antiplatelet action of aspirin and its role in thrombosis treatment and prophylaxis.[Pubmed:9263351]
Semin Thromb Hemost. 1997;23(4):349-56.
The antithrombotic action of Aspirin (Acetylsalicylic acid) is due to inhibition of platelet function by acetylation of the platelet cyclooxygenase (COX) at the functionally important amino acid serine529. This prevents the access of the substrate (arachidonic aid) to the catalytic site of the enzyme at tyrosine385 and results in an irreversible inhibition of platelet-dependent thromboxane formation. Aspirin is an approximately 150- to 200-fold more potent inhibitor of the (constitutive) isoform of the platelet enzyme (COX-1) than the (inducible) isoform (COX-2) which is expressed by cytokines, inflammatory stimuli, and some growth factors. This explains the different dosage requirements of aspirin as an antithrombotic (COX-1) and an anti-inflammatory drug (COX-2), respectively. Aspirin is the "gold standard" antiplatelet agent for prevention of arterial thromboses. The optimum dose of aspirin as an antithrombotic drug can differ in different organ circulations. While 100 mg/day is sufficient for prevention of thrombus formation in the coronary circulation, higher doses may be required for the prevention of vascular events in the cerebral and peripheral circulation. However, any effective antiplatelet dose of aspirin is associated with an increased risk of bleeding. Therefore, the individual benefit/risk ratio determines the administration of the compound. There are no known prostaglandin-independent mechanisms for the antithrombotic action of aspirin in clinical use. Thus, platelet activation caused by other factors remains unchanged and might result in a resistance against inhibition of platelet function by aspirin. This involves platelet activation by shear stress and ADP. Additionally, there is no "sparing" of endothelial prostacyclin synthesis in clinical conditions of atherosclerotic endothelial injury. In this case, inhibition of COX-1 by aspirin will also reduce the amount of precursors for vascular prostacyclin synthesis, provided, for example, from adhering platelets.


