Ascorbic acidEnhances the generation of iPSCs; increases reprogramming efficiency CAS# 50-81-7 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 50-81-7 | SDF | Download SDF |
| PubChem ID | 54670067 | Appearance | Powder |
| Formula | C6H8O8 | M.Wt | 208.1 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | L-Ascorbate, Vitamin C | ||
| Solubility | Soluble to 500 mM in water and to 100 mM in DMSO | ||
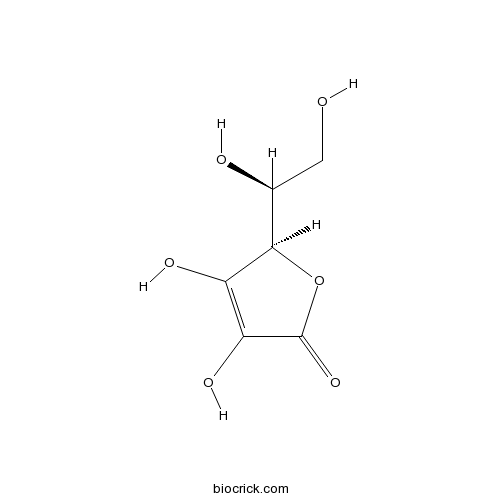
| Chemical Name | (2R)-2-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxy-2H-furan-5-one | ||
| SMILES | C(C(C1C(=C(C(=O)O1)O)O)O)O | ||
| Standard InChIKey | CIWBSHSKHKDKBQ-JLAZNSOCSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ascorbic acid (Vitamin C) is a water-soluble vitamin indicated for the prevention and treatment of scurvy, it has a protective effect on alloxan-induced damage by maintaining the activity of cellular antioxidants, it also protects sperm from endogenous oxidative DNA damage that could affect sperm quality and increases risk of genetic defects, particularly in populations with low ascorbic acid such as smokers.Ascorbic acid can reverse endothelial vasomotor dysfunction in the brachial circulation of patients with coronary artery disease. |
| Targets | p53 | DNA/RNA Synthesis | Mdm2 |
| In vivo | α-Tocopherol, ascorbic acid, and β-carotene protect against oxidative stress but reveal no direct influence on p53 expression in rats subjected to stress.[Pubmed: 24074745]Nutr Res. 2013 Oct;33(10):868-75.We hypothesized that α-tocopherol, Ascorbic acid, and β-carotene, either applied individually or in combination, would modulate redox homeostasis and affect the regulation of genes involved in DNA repair under stress conditions.
Effect of intravenous ascorbic acid in hemodialysis patients with EPO-hyporesponsive anemia and hyperferritinemia.[Pubmed: 16564942 ]Am J Kidney Dis. 2006 Apr;47(4):644-54.Although erythropoietin (EPO)-hyporesponsive anemia in hemodialysis patients most commonly results from iron deficiency, the contributory role of chronic inflammation and oxidative stress in its pathogenesis is poorly understood. We conducted an open-label prospective study to assess the effect of vitamin C(Ascorbic acid), an antioxidant, on EPO-hyporesponsive anemia in hemodialysis patients with unexplained hyperferritinemia.
Enhanced testicular antioxidant system by ascorbic acid in alloxan diabetic rats.[Pubmed: 10661714]Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999 Nov;124(3):233-7.The diabetic subject is at significantly increased risk of developing testicular changes. Its etiology may involve oxidative damage by free radicals and protection against such damage can be offered by antioxidant supplementation.
Ascorbic acid protects against endogenous oxidative DNA damage in human sperm.[Pubmed: 1763015]Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11003-6.Damage to the DNA of germ cells can lead to mutation, which may result in birth defects, genetic diseases, and cancer.
The very high endogenous rate of oxidative DNA damage and the importance of dietary Ascorbic acid (AA) in preventing this damage has prompted an examination of these factors in human sperm DNA.
Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease.[Pubmed: 8653830]Circulation. 1996 Mar 15;93(6):1107-13.In the setting of atherosclerosis, endothelial vasomotor function is abnormal. Increased oxidative stress has been implicated as one potential mechanism for this observation. We therefore hypothesized that an antioxidant, Ascorbic acid, would improve endothelium-dependent arterial dilation in patients with coronary artery disease.
|
| Animal Research | Dietary ascorbic acid needs of common carp (Cyprinus carpio) larvae.[Reference: WebLink]Aquaculture, 1998, 161(1–4):453-61.As some controversy seem to exist regarding the dietary essentiality of Ascorbic acid (AA) for larval cyprinids, a study was conducted to determine the dietary AA requirements with first-feeding larvae of common carp.
|

Ascorbic acid Dilution Calculator

Ascorbic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8054 mL | 24.0269 mL | 48.0538 mL | 96.1076 mL | 120.1346 mL |
| 5 mM | 0.9611 mL | 4.8054 mL | 9.6108 mL | 19.2215 mL | 24.0269 mL |
| 10 mM | 0.4805 mL | 2.4027 mL | 4.8054 mL | 9.6108 mL | 12.0135 mL |
| 50 mM | 0.0961 mL | 0.4805 mL | 0.9611 mL | 1.9222 mL | 2.4027 mL |
| 100 mM | 0.0481 mL | 0.2403 mL | 0.4805 mL | 0.9611 mL | 1.2013 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ascorbic acid is a naturally occurring organic compound with antioxidant properties.
- Aspirin (Acetylsalicylic acid)
Catalog No.:BCC2097
CAS No.:50-78-2
- Actinomycin D
Catalog No.:BCC2385
CAS No.:50-76-0
- 1,3:2,4-Di-p-methylbenyliedene sorbitol
Catalog No.:BCC4847
CAS No.:54686-97-4
- 5-Hydroxytryptamine
Catalog No.:BCC9204
CAS No.:50-67-9
- Niclosamide
Catalog No.:BCC5081
CAS No.:50-65-7
- Chloroquine diphosphate
Catalog No.:BCC3915
CAS No.:50-63-5
- Oxytocin
Catalog No.:BCC5435
CAS No.:50-56-6
- Reserpine
Catalog No.:BCN4960
CAS No.:50-55-5
- Estradiol Benzoate
Catalog No.:BCC4779
CAS No.:50-50-0
- Mercaptopurine (6-MP)
Catalog No.:BCC1186
CAS No.:50-44-2
- Clomiphene citrate
Catalog No.:BCC4480
CAS No.:50-41-9
- Cocaine
Catalog No.:BCN1901
CAS No.:50-36-2
- Thymidine
Catalog No.:BCN5622
CAS No.:50-89-5
- Floxuridine
Catalog No.:BCC1187
CAS No.:50-91-9
- Ephedrine Hydrochloride
Catalog No.:BCC8322
CAS No.:50-98-6
- D-(+)-Glucose
Catalog No.:BCN1259
CAS No.:50-99-7
- Nordihydroguaiaretic acid
Catalog No.:BCC1805
CAS No.:500-38-9
- L-Mimosine
Catalog No.:BCC5450
CAS No.:500-44-7
- Apoatropine
Catalog No.:BCN1869
CAS No.:500-55-0
- Convolamine
Catalog No.:BCN1905
CAS No.:500-56-1
- Yangonin
Catalog No.:BCN3565
CAS No.:500-62-9
- Kawain
Catalog No.:BCN3564
CAS No.:500-64-1
- Rhapontigenin
Catalog No.:BCN3515
CAS No.:500-65-2
- Olivetol
Catalog No.:BCN4629
CAS No.:500-66-3
Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease.[Pubmed:8653830]
Circulation. 1996 Mar 15;93(6):1107-13.
BACKGROUND: In the setting of atherosclerosis, endothelial vasomotor function is abnormal. Increased oxidative stress has been implicated as one potential mechanism for this observation. We therefore hypothesized that an antioxidant, Ascorbic acid, would improve endothelium-dependent arterial dilation in patients with coronary artery disease. METHODS AND RESULTS: Brachial artery endothelium-dependent dilation in response to hyperemia was assessed by high-resolution vascular ultrasound before and 2 hours after oral administration of either 2 g Ascorbic acid or placebo in a total of 46 patients with documented coronary artery disease. Plasma Ascorbic acid concentration increased 2.5-fold 2 hours after treatment (46+/-8 to 114+/-11 micromol/L, P=.001). In the prospectively defined group of patients with an abnormal baseline response (<5% dilation), Ascorbic acid produced marked improvement in dilation (2.0+/-0.6% to 9.7+/-2.0%), whereas placebo had no effect (1.1+/-1.5% to 1.7+/-1.5%, P=.003 for Ascorbic acid versus placebo). Ascorbic acid had no effect on hyperemic flow or arterial dilation to sublingual nitroglycerin. CONCLUSIONS: Ascorbic acid reverses endothelial vasomotor dysfunction in the brachial circulation of patients with coronary artery disease. These findings suggest that increased oxidative stress contributes to endothelial dysfunction in patients with atherosclerosis and that endothelial dysfunction may respond to antioxidant therapy.
Effect of intravenous ascorbic acid in hemodialysis patients with EPO-hyporesponsive anemia and hyperferritinemia.[Pubmed:16564942]
Am J Kidney Dis. 2006 Apr;47(4):644-54.
BACKGROUND: Although erythropoietin (EPO)-hyporesponsive anemia in hemodialysis patients most commonly results from iron deficiency, the contributory role of chronic inflammation and oxidative stress in its pathogenesis is poorly understood. We conducted an open-label prospective study to assess the effect of vitamin C, an antioxidant, on EPO-hyporesponsive anemia in hemodialysis patients with unexplained hyperferritinemia. METHODS: Forty-six of 262 patients in an inner-city hemodialysis center met the inclusion criteria (administration of intravenous iron and EPO for > or = 6 months at a dose > or = 450 U/kg/wk, average 3-month hemoglobin [Hb] level < or = 11.0 g/dL [< or = 110 g/L], ferritin level > or = 500 ng/mL (microg/L), and transferrin saturation [TSAT] < or = 50%). Patients were excluded if they had a clear explanation for the EPO hyporesponsiveness. Four patients refused to participate. The remaining patients were randomly assigned; 20 patients to receive standard care and 300 mg of intravenous vitamin C with each dialysis session (group 1) and 22 patients to receive standard care only (group 2). Study duration was 6 months. During the study, 1 patient from group 1 was removed (upper gastrointestinal bleeding) from final analysis. Monthly assessment included Hb level, mean corpuscular volume, iron level, iron-binding capacity, ferritin level, TSAT, and Hb content in reticulocytes. In addition, biointact parathyroid hormone, aluminum, C-reactive protein (CRP), and liver enzymes were measured every 3 months. RESULTS: Age, sex, race, and time on dialysis therapy were similar in both groups. At 6 months, Hb levels significantly increased from 9.3 to 10.5 g/dL (93.0 to 105.0 g/L) in group 1, but not group 2 (9.3 to 9.6 g/dL [93.0 to 96.0 g/L]; P = 0.0001). Similarly, TSAT increased from 28.9% to 37.3% in group 1, but not group 2 (28.7% to 29.3%; P = 0.0001). EPO dose (477 to 429 versus 474 to 447 U/kg/wk), iron-binding capacity (216 to 194 versus 218 to 257 microg/dL [38.7 to 34.7 versus 39 to 46 micromol/L]), and CRP level (2.8 to 0.9 versus 2.8 to 2.2 mg/dL) decreased significantly in group 1, but not in controls. Changes in Hb content in reticulocytes and ferritin level also were statistically significant in group 1. There was no change in biointact parathyroid hormone levels. Although serum iron levels and intravenous iron doses changed within each group, changes were equal between the 2 groups. CONCLUSION: In hemodialysis patients with refractory anemia and hyperferritinemia, vitamin C improved responsiveness to EPO, either by augmenting iron mobilization from its tissue stores or through antioxidant effects.
Ascorbic acid recycling enhances the antioxidant reserve of human erythrocytes.[Pubmed:7548025]
Biochemistry. 1995 Oct 3;34(39):12721-8.
The role of ascorbate transport and metabolism in the response of human erythrocytes to an extracellular oxidant stress was investigated. Rates of entry and exit of [14C]dehydroascorbate from erythrocytes were more than 10-fold greater than those of [14C]ascorbate. Both the reduced and oxidized forms of the vitamin were transported largely by the glucose transporter. Inside erythrocytes, dehydroascorbate was converted to ascorbate, increasing intracellular ascorbate concentrations 2-3-fold over those in the medium. In such ascorbate-loaded cells, the membrane-impermeant oxidant ferricyanide induced a transmembrane oxidation of intracellular ascorbate to dehydroascorbate. The latter escaped the cells on the glucose transporter, which resulted in a halving of the net entry of [14C]dehydroascorbate in the presence of ferricyanide. Treatment of ascorbate-loaded cells with H2O2 and Cu2+ also oxidized ascorbate and induced efflux of [14C]dehydroascorbate. Ferricyanide-dependent intracellular oxidation of ascorbate resulted in a corresponding reduction of extracellular ferricyanide, which served as an integrated measure of ascorbate recycling. Ferricyanide reduction was proportional to the loading concentration of dehydroascorbate and was enhanced when loss of dehydroascorbate from cells was decreased, either by blockade of the glucose transporter or by concentrating the cells. Selective depletion of cellular ascorbate lowered rates of ferricyanide reduction by two-thirds, suggesting that ascorbate rather than NADH is the major donor for the transmembrane ferricyanide oxidoreductase activity. On the basis of the ascorbate-dependent rate of ferricyanide reduction, erythrocytes at a 45% hematocrit can regenerate the Ascorbic acid present in whole blood every 3 min. Erythrocyte ascorbate recycling may thus contribute more to the antioxidant reserve of blood than is evident from plasma ascorbate concentrations alone.
alpha-Tocopherol, ascorbic acid, and beta-carotene protect against oxidative stress but reveal no direct influence on p53 expression in rats subjected to stress.[Pubmed:24074745]
Nutr Res. 2013 Oct;33(10):868-75.
We hypothesized that alpha-tocopherol, Ascorbic acid, and beta-carotene, either applied individually or in combination, would modulate redox homeostasis and affect the regulation of genes involved in DNA repair under stress conditions. To test this hypothesis, we analyzed the influence of these vitamins, either supplied individually or in combination, on the plasma lipid peroxide level and the hepatic level of 8-hydroxy-2'-deoxyguanosine in rats. We also evaluated the expression of p53 and Mdm2 protein in the intestinal epithelium, as these proteins are involved in the cellular regulation of DNA damage repair. Male Wistar rats (n = 112) were supplemented with alpha-tocopherol (2 mg), Ascorbic acid (12 mg), and beta-carotene (1 mg), both individually and in combination, for 14 days; 32 control rats were treated with placebo. Half of the animals in each group (n = 8) were subjected to 15-minute treadmill running at 20 m/min to cause exercise-induced oxidative stress. A statistically significant reduction in lipid peroxide levels was observed in the plasma of rats subjected to exercise and given 2 or 3 of the antioxidants (P < .0001). Exercise, as well as coadministration of the antioxidants, had no significant effect on the amount of DNA damage. Downward trends in the level of p53 protein expression were observed both in exercised and nonexercised animals, especially when the studied vitamins were administered in combination. Our findings suggest that alpha-tocopherol, Ascorbic acid, and beta-carotene, when given concurrently, have primarily antioxidant effects on lipids under stress but do not significantly affect the regulation of p53 gene expression.
Ascorbic acid protects against endogenous oxidative DNA damage in human sperm.[Pubmed:1763015]
Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11003-6.
Damage to the DNA of germ cells can lead to mutation, which may result in birth defects, genetic diseases, and cancer. The very high endogenous rate of oxidative DNA damage and the importance of dietary Ascorbic acid (AA) in preventing this damage has prompted an examination of these factors in human sperm DNA. The oxidized nucleoside 8-hydroxy-2'-deoxyguanosine (8-oxo-7,8-dihydro-2'-deoxyguanosine; oxo8dG), 1 of approximately 20 major products of oxidative damage to DNA, was measured in DNA isolated from human sperm provided by healthy subjects and compared to the seminal fluid AA levels. This relationship was studied in two groups. In a group of 24 free-living individuals 20-50 years old high levels of oxo8dG were correlated with low seminal plasma AA. The endogenous level of oxo8dG in this group was 13 fmol per microgram of DNA or approximately 25,000 adducts per sperm cell. The second group of individuals was maintained on a controlled diet that varied only in AA content. When dietary AA was decreased from 250 to 5 mg/day, the seminal fluid AA decreased by half and the level of oxo8dG in sperm DNA increased 91%. Repletion of dietary AA for 28 days (from 5 mg/day to 250 or 60 mg/day) caused a doubling in seminal fluid AA and reduced oxo8dG by 36%. These results indicate that dietary AA protects human sperm from endogenous oxidative DNA damage that could affect sperm quality and increase risk of genetic defects, particularly in populations with low AA such as smokers.
Enhanced testicular antioxidant system by ascorbic acid in alloxan diabetic rats.[Pubmed:10661714]
Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999 Nov;124(3):233-7.
The diabetic subject is at significantly increased risk of developing testicular changes. Its etiology may involve oxidative damage by free radicals and protection against such damage can be offered by antioxidant supplementation. Alloxan elicited significant inhibition of antioxidants including superoxide dismutase, catalase and glutathione reductase activities and decreased glutathione content in testis. These effects were accompanied by significant elevation of testicular lipid peroxidation, decreased plasma testosterone level and a drop in copper and zinc concentrations in testis. The administration of Ascorbic acid after alloxan treatment interfered and prevented alloxan action. Ascorbic acid blunted the increased testicular lipid peroxidation and the decreased plasma testosterone level probably by protecting antioxidants and the loss of copper and zinc from testes. The data suggested that Ascorbic acid has a protective effect on alloxan-induced damage by maintaining the activity of cellular antioxidants.
Vitamin C enhances the generation of mouse and human induced pluripotent stem cells.[Pubmed:20036631]
Cell Stem Cell. 2010 Jan 8;6(1):71-9.
Somatic cells can be reprogrammed into induced pluripotent stem cells (iPSCs) by defined factors. However, the low efficiency and slow kinetics of the reprogramming process have hampered progress with this technology. Here we report that a natural compound, vitamin C (Vc), enhances iPSC generation from both mouse and human somatic cells. Vc acts at least in part by alleviating cell senescence, a recently identified roadblock for reprogramming. In addition, Vc accelerates gene expression changes and promotes the transition of pre-iPSC colonies to a fully reprogrammed state. Our results therefore highlight a straightforward method for improving the speed and efficiency of iPSC generation and provide additional insights into the mechanistic basis of the reprogramming process.
Molecular mechanisms of subtype-specific inhibition of neuronal T-type calcium channels by ascorbate.[Pubmed:18003836]
J Neurosci. 2007 Nov 14;27(46):12577-83.
T-type Ca2+ channels (T-channels) are involved in the control of neuronal excitability and their gating can be modulated by a variety of redox agents. Ascorbate is an endogenous redox agent that can function as both an anti- and pro-oxidant. Here, we show that ascorbate selectively inhibits native Ca(v)3.2 T-channels in peripheral and central neurons, as well as recombinant Ca(v)3.2 channels heterologously expressed in human embryonic kidney 293 cells, by initiating the metal-catalyzed oxidation of a specific, metal-binding histidine residue in domain 1 of the channel. Our biophysical experiments indicate that ascorbate reduces the availability of Ca(v)3.2 channels over a wide range of membrane potentials, and inhibits Ca(v)3.2-dependent low-threshold-Ca2+ spikes as well as burst-firing in reticular thalamic neurons at physiologically relevant concentrations. This study represents the first mechanistic demonstration of ion channel modulation by ascorbate, and suggests that ascorbate may function as an endogenous modulator of neuronal excitability.
Vitamin C as an antioxidant: evaluation of its role in disease prevention.[Pubmed:12569111]
J Am Coll Nutr. 2003 Feb;22(1):18-35.
Vitamin C in humans must be ingested for survival. Vitamin C is an electron donor, and this property accounts for all its known functions. As an electron donor, vitamin C is a potent water-soluble antioxidant in humans. Antioxidant effects of vitamin C have been demonstrated in many experiments in vitro. Human diseases such as atherosclerosis and cancer might occur in part from oxidant damage to tissues. Oxidation of lipids, proteins and DNA results in specific oxidation products that can be measured in the laboratory. While these biomarkers of oxidation have been measured in humans, such assays have not yet been validated or standardized, and the relationship of oxidant markers to human disease conditions is not clear. Epidemiological studies show that diets high in fruits and vegetables are associated with lower risk of cardiovascular disease, stroke and cancer, and with increased longevity. Whether these protective effects are directly attributable to vitamin C is not known. Intervention studies with vitamin C have shown no change in markers of oxidation or clinical benefit. Dose concentration studies of vitamin C in healthy people showed a sigmoidal relationship between oral dose and plasma and tissue vitamin C concentrations. Hence, optimal dosing is critical to intervention studies using vitamin C. Ideally, future studies of antioxidant actions of vitamin C should target selected patient groups. These groups should be known to have increased oxidative damage as assessed by a reliable biomarker or should have high morbidity and mortality due to diseases thought to be caused or exacerbated by oxidant damage.


