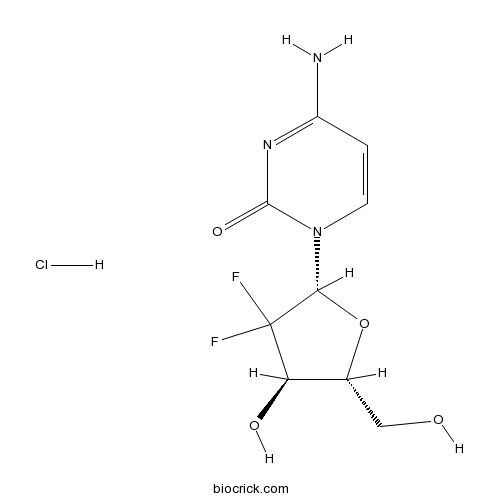
Gemcitabine HClInhibits DNA synthesis,deoxycytidine analog CAS# 122111-03-9 |
- CX-5461
Catalog No.:BCC3700
CAS No.:1138549-36-6
- Fludarabine
Catalog No.:BCC2518
CAS No.:21679-14-1
- Carboplatin
Catalog No.:BCC1170
CAS No.:41575-94-4
- Epirubicin HCl
Catalog No.:BCC1192
CAS No.:56390-09-1
- Fludarabine Phosphate (Fludara)
Catalog No.:BCC3681
CAS No.:75607-67-9
- Bleomycin Sulfate
Catalog No.:BCC3694
CAS No.:9041-93-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 122111-03-9 | SDF | Download SDF |
| PubChem ID | 60749 | Appearance | Powder |
| Formula | C9H12ClF2N3O4 | M.Wt | 299.66 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GEMCITABINE HYDROCHLORIDE;Gemzar; Dizirconium silicide | ||
| Solubility | H2O : ≥ 66.66 mg/mL (222.45 mM) DMSO : 46.67 mg/mL (155.74 mM; Need ultrasonic) DMF : 2.5 mg/mL (8.34 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (+)-2'-Deoxy-2',2'-difluorocytidine hydrochloride | ||
| SMILES | [Cl-].NC1=NC(=O)N(C=C1)[C@@H]2O[C@H](CO)[C@@H](O)C2(F)F.[H+] | ||
| Standard InChIKey | OKKDEIYWILRZIA-OSZBKLCCSA-N | ||
| Standard InChI | InChI=1S/C9H11F2N3O4.ClH/c10-9(11)6(16)4(3-15)18-7(9)14-2-1-5(12)13-8(14)17;/h1-2,4,6-7,15-16H,3H2,(H2,12,13,17);1H/t4-,6-,7-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Deoxycytidine analog that inhibits DNA synthesis. Metabolized to form gemcitabine triphosphate (dFdCTP) and gemcitabine diphosphate (dFdCDP). dFdCTD inhibits ribonucleotide reductase causing a reduction in cellular nucleotides. dFdCTP is incorporated in DNA resulting in DNA strand termination. Displays antitumor activity in vitro and in vivo. |

Gemcitabine HCl Dilution Calculator

Gemcitabine HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3371 mL | 16.6856 mL | 33.3712 mL | 66.7423 mL | 83.4279 mL |
| 5 mM | 0.6674 mL | 3.3371 mL | 6.6742 mL | 13.3485 mL | 16.6856 mL |
| 10 mM | 0.3337 mL | 1.6686 mL | 3.3371 mL | 6.6742 mL | 8.3428 mL |
| 50 mM | 0.0667 mL | 0.3337 mL | 0.6674 mL | 1.3348 mL | 1.6686 mL |
| 100 mM | 0.0334 mL | 0.1669 mL | 0.3337 mL | 0.6674 mL | 0.8343 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
An UV spectrophotometry-based method accurately, reliably and rapidly determines GMCT with low cost and simple procedures.
Abstract
As an antitumor agent, Gemcitabine HCL, which induces apoptosis through caspase activation, exhibited lower cytotoxicity in CCRF-CEM-AraC-8C, CCRF-CEM/dCK(-/-) and TC-1-GR cell lines but higher cytotoxicity in wild type CCRF-CEM cell lines than GemC18-NPs.
Abstract
Daily oral administration of LY2334737 of 6 mg/kg for 21 days is equivalent to weekly i.v. administration of gemcitabine HCL of 240 mg/kg for 3 weeks in mice bearing a patient mesothelioma tumor PXF 1118 or a non-small cell lung cancer tumor LXFE 927.
Abstract
Gemcitabine HCL is an approved antitumor agent that has lower cytotoxicity than monophosphorylated gemcitabine derivatives in dCK deficient cells and cells with overexpressing RRM1 or RRM2.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Gemcitabine HCl is an inhibitor of DNA synthesis with the IC50 value of 240.4±29.0 μM (CCRF-CEM/dCK−/− cells), 14.7±2.8 nM (TC-1 cells), 36.7 ± 5.1 μM (TC-1-GR cells), and 50 nM (PANC1 cells) [1].
DNA synthesis is the natural or artificial creation of DNA molecules and can be defined as DNA replication, polymerase chain reaction, and gene synthesis. It is reported that DNA synthesis process plays an important role in a variety of cancers, many drugs have been made to target this process to inhibit tumor growth or metastasis [2] [3].
Gemcitabine HCl is a DNA synthesis inhibitor. When tested with pancreatic cancer cell line COLO 357 and L3.6pl, Gemcitabine HCl treatment following genistein which sensitized cells to Gemcitabine HCL significantly inhibited cell growth and increased cell apoptosis [4]. In MIA PaCa-2 cells, Gemcitabine HCl showed markedly cytotoxicity to cells with the IC50 value of 49.7 ± 17.7 nM via inhibiting the activity of dDNA [1].
In SCID mice bearing COLO 357 cells orthotopically implanted, compared with control group, treated both genistein and gemcitabine significantly decreased (75%, P < 0.01) tumor growth and body weight [4].
References:
[1].Lansakara, P.D., B.L. Rodriguez, and Z. Cui, Synthesis and in vitro evaluation of novel lipophilic monophosphorylated gemcitabine derivatives and their nanoparticles. Int J Pharm, 2012. 429(1-2): p. 123-34.
[2].Kostyrev, O.A. and T.A. Leont'eva, [Autoradiographic study of DNA systhesis in muscle and connective tissue cells of the heart during exposure to isopropylnorepinephrine]. Biull Eksp Biol Med, 1973. 76(7): p. 108-11.
[3].Mathews, L.A., et al., Increased expression of DNA repair genes in invasive human pancreatic cancer cells. Pancreas, 2011. 40(5): p. 730-9.
[4].Banerjee, S., et al., Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res, 2005. 65(19): p. 9064-72.
- 2-Deoxy-2,2-difluoro-D-erythro-pentafuranous-1-ulose-3,5-dibenzoate
Catalog No.:BCC8575
CAS No.:122111-01-7
- Auraptenol
Catalog No.:BCN6113
CAS No.:1221-43-8
- 3,4-Dihydro-3,4-dihydroxynaphthalen-1(2H)-one
Catalog No.:BCN1602
CAS No.:1220891-22-4
- Charantadiol A
Catalog No.:BCN3483
CAS No.:1220890-23-2
- 2'-O-Acetylsprengerinin C
Catalog No.:BCN6655
CAS No.:1220707-33-4
- [bAla8]-Neurokinin A(4-10)
Catalog No.:BCC7137
CAS No.:122063-01-8
- Khayalenoid E
Catalog No.:BCN6111
CAS No.:1220508-29-1
- Monomethyl lithospermate B
Catalog No.:BCN2533
CAS No.:122021-74-3
- PLP (139-151)
Catalog No.:BCC5920
CAS No.:122018-58-0
- Cyhalofop
Catalog No.:BCC5474
CAS No.:122008-78-0
- Niazirin
Catalog No.:BCN7300
CAS No.:122001-32-5
- Sulfamonomethoxine
Catalog No.:BCC9156
CAS No.:1220-83-3
- ER-878898
Catalog No.:BCC8958
CAS No.:122111-11-9
- Acarbose sulfate
Catalog No.:BCC4284
CAS No.:1221158-13-9
- Dehydroborapetoside B
Catalog No.:BCN6601
CAS No.:1221178-16-0
- ML 202
Catalog No.:BCC6306
CAS No.:1221186-52-2
- Meliasenin B
Catalog No.:BCN6112
CAS No.:1221262-77-6
- BP 554 maleate
Catalog No.:BCC6695
CAS No.:1221401-95-1
- Skepinone-L
Catalog No.:BCC1953
CAS No.:1221485-83-1
- Gymnemic acid I
Catalog No.:BCN2679
CAS No.:122168-40-5
- NPS-1034
Catalog No.:BCC6504
CAS No.:1221713-92-3
- 5'-Geranyl-5,7,2',4'-tetrahydroxyflavone
Catalog No.:BCN1601
CAS No.:1221762-70-4
- Boc-Dap(Fmoc)-OH
Catalog No.:BCC2665
CAS No.:122235-70-5
- 3-Epimeliasenin B
Catalog No.:BCN4723
CAS No.:1222475-77-5
Design and evaluation of an intravesical delivery system for superficial bladder cancer: preparation of gemcitabine HCl-loaded chitosan-thioglycolic acid nanoparticles and comparison of chitosan/poloxamer gels as carriers.[Pubmed:26508855]
Int J Nanomedicine. 2015 Oct 14;10:6493-507.
This study aimed to develop an intravesical delivery system of Gemcitabine HCl for superficial bladder cancer in order to provide a controlled release profile, to prolong the residence time, and to avoid drug elimination via urination. For this aim, bioadhesive nanoparticles were prepared with thiolated chitosan (chitosan-thioglycolic acid conjugate) and were dispersed in bioadhesive chitosan gel or in an in situ gelling poloxamer formulation in order to improve intravesical residence time. In addition, nanoparticle-loaded gels were diluted with artificial urine to mimic in vivo conditions in the bladder and were characterized regarding changes in gel structure. The obtained results showed that chitosanthioglycolic acid nanoparticles with a mean diameter of 174.5+/-3.762 nm and zeta potential of 32.100+/-0.575 mV were successfully developed via ionotropic gelation and that the encapsulation efficiency of Gemcitabine HCl was nearly 20%. In vitro/ex vivo characterization studies demonstrated that both nanoparticles and nanoparticle-loaded chitosan and poloxamer gels might be alternative carriers for intravesical administration of Gemcitabine HCl, prolonging its residence time in the bladder and hence improving treatment efficacy. However, when the gel formulations were diluted with artificial urine, poloxamer gels lost their in situ gelling properties at body temperature, which is in conflict with the aimed formulation property. Therefore, 2% chitosan gel formulation was found to be a more promising carrier system for intravesical administration of nanoparticles.
Inhalable liposomal dry powder of gemcitabine-HCl: Formulation, in vitro characterization and in vivo studies.[Pubmed:26453787]
Int J Pharm. 2015 Dec 30;496(2):886-95.
Pulmonary drug delivery system facilitates local instillation of anticancer drugs to lungs which has proven to be pioneering approach for treatment of lung cancer. This approach led the groundwork for delivering liposomal formulation directly to lungs. Gemcitabine-HCl is currently considered as most effective drug for management of lung cancer. However, its application is limited owing to its metabolism by enzymes present in plasma resulting in reduced efficacy and higher toxicity. In present study, lyophilisation technique was used to convert liposomes into dry powder inhaler, which was formulated using emulsification solvent evaporation technique. The physicochemical properties including size, morphology, entrapment efficiency, loading efficiency etc. of formulated liposomes were evaluated. The prepared liposomal DPI (LDPI) formulations were then examined for solid state characteristics and aerosol performance using cascade impactor. From all the formulations prepared, the LDPI formulated using trehalose as cryoprotectant presented required properties along with desirable deposition pattern. Finally, the optimized formulation was selected for in vitro cell line studies; in vivo studies and stability study. This formulated inhalable particles offers a promising approach for the management of lung cancer through regional chemotherapy.
Counter-intuitive effect of non-crystallizing sugars on the crystallization of gemcitabine HCl in frozen solutions.[Pubmed:25445978]
Int J Pharm. 2015 Jan 15;478(1):46-52.
In this study, the effect of four non-crystallizing sugars, namely fructose, trehalose, sucrose and raffinose, was assessed on the crystallization of gemcitabine hydrochloride (GHCl) in frozen solutions. Aqueous solutions containing GHCl (50 mg/mL) and a sugar at varying concentrations (10-60 mg/mL) were frozen in situ in DSC and analyzed in the subsequent heating run. Crystallization propensity of GHCl was quantified in terms of reduced crystallization temperature (RCT) as a function of sugar type and concentration. Multivariate analysis option in JMP((R)) software was employed for calculating correlation between the variables. All sugars inhibited GHCl crystallization in a concentration dependent manner. At equal concentration, fructose (with the lowest Tg') exerted the strongest inhibitory effect, whereas raffinose (with the highest Tg') exerted the weakest inhibitory effect. Additionally, RCT showed a poor correlation with Tg' (r=0.2327). Thus, the inhibitory effect of sugars could not be described by their anti-plasticization effect. This counter-intuitive behavior was explained by the inhibitory effect of sugars on ice crystallization, which increased the unfrozen water content (UWC) in the freeze concentrate, thereby lowering the supersaturation of GHCl. This was established by observing a good correlation (r=0.9666) between RCT and ln(1/UWC). Additionally, reduced diffusion kinetics of GHCl in presence of sugar molecules was also postulated. This study highlights the importance of unfrozen water towards governing the crystallization behavior of solutes in multi-component frozen systems.
A phase I pilot study of the insulin-like growth factor 1 receptor pathway modulator AXL1717 in combination with gemcitabine HCl and carboplatin in previously untreated, locally advanced, or metastatic non-small cell lung cancer.[Pubmed:25794491]
Med Oncol. 2015 Apr;32(4):129.
AXL1717 is an orally bioavailable IGF-1R pathway modulator that has been shown to have anti-tumoral effects. The objectives of the present study were to define maximum tolerated dose and the recommended phase II dose (RPTD) of AXL1717 in combination with Gemcitabine HCl and carboplatin in non-small cell lung cancer (NSCLC). Patients with previously untreated, locally advanced, or metastatic NSCLC (squamous cell cancer or adenocarcinoma) in good performance status and with preserved major organ functions were enrolled in the study. The study was an open-label phase I study with planned cohorts of three patients per dose level of AXL1717 (215, 290, and 390 mg BID). In total, 12 patients were enrolled in the study, and of these, two were prematurely excluded. AXL1717 was administered at one dose level, 215 mg BID. A total number of 81 unique adverse events were reported. Bone marrow toxicity was reported in 10 out of 12 patients, and this organ class showed the largest number of related events. AXL1717 in combination with Gemcitabine HCl and carboplatin is a possible treatment approach in previously untreated, locally advanced, or metastatic non-small cell lung cancer. However, due to the bone marrow toxicity profile shown in the present study, further dose increases of AXL1717 above 215 mg BID will probably not be feasible. Therefore, 215 mg BID constitutes maximum tolerated dose and RPTD.
Gemcitabine: a modulator of intracellular nucleotide and deoxynucleotide metabolism.[Pubmed:7481839]
Semin Oncol. 1995 Aug;22(4 Suppl 11):11-8.
Gemcitabine (2',2'-difluorodeoxycytidine, dFdC) is a deoxycytidine (dCyd) analog that extensively modulates intracellular CTP and dCTP metabolism. In Chinese hamster ovary (CHO) cells, a 4-hour exposure to gemcitabine (100 mumol/L) reduced cellular CTP and dCTP concentrations to 5.9% and 50%, respectively. Intracellular UTP concentrations increased, indicating a metabolic block at CTP synthetase. Pool-sizes of ATP and GTP remained unaffected. In contrast, a CHO mutant deficient in deoxycytidine kinase, and thus unable to accumulate dFdCTP, maintained its CTP pools under identical conditions, suggesting that the CTP pool depletion was dependent on dFdC phosphorylation. Neither 100 mumol/L arabinosylcytosine nor 5 mmol/L hydroxyurea affected CTP levels, indicating that inhibition of DNA synthesis by analog incorporation or by depletion of dNTP pools were not the causes of the CTP pool perturbation. Metabolic studies demonstrated that incorporation of [3H]uridine into the UTP pool was not impaired by dFdC treatment, whereas the specific activity of the CTP pools decreased as a function of increasing gemcitabine concentration and time of exposure. Comparable results were obtained using 3-deazauridine, a known inhibitor of CTP synthetase. We conclude that high cellular concentrations of dFdCTP deplete cellular CTP concentrations by inhibition of the dCTP pool and also may be a limiting factor for RNA synthesis.
Preclinical characteristics of gemcitabine.[Pubmed:8718419]
Anticancer Drugs. 1995 Dec;6 Suppl 6:7-13.
Gemcitabine (2',2'-difluorodeoxycytidine, dFdC) is a nucleoside analogue of deoxycytidine in which two fluorine atoms have been inserted into the deoxyribofuranosyl ring. Once inside the cell gemcitabine is rapidly phosphorylated by deoxycytidine kinase, the rate-limiting enzyme for the formation of the active metabolites gemcitabine diphosphate (dFdCDP) and gemcitabine triphosphate (dFdCTP). Gemcitabine diphosphate inhibits ribonucleotide reductase, which is responsible for producing the deoxynucleotides required for DNA synthesis and repair. The subsequent decrease in cellular deoxynucleotides (particularly dCTP) favours gemcitabine triphosphate in its competition with dCTP for incorporation into DNA. Reduction in cellular dCTP is an important self-potentiating mechanism resulting in increased gemcitabine nucleotide incorporation into DNA. Other self-potentiating mechanisms of gemcitabine include increased formation of active gemcitabine di- and triphosphates, and decreased elimination of gemcitabine nucleotides. After gemcitabine nucleotide is incorporated on the end of the elongating DNA strand, one more deoxynucleotide is added, and thereafter the DNA polymerases are unable to proceed. This action, termed "masked chain termination", appears to lock the drug into DNA because proof-reading exonucleases are unable to remove gemcitabine nucleotide from this penultimate position. Incorporation of gemcitabine triphosphate into DNA is strongly correlated with the inhibition of further DNA synthesis. Compared with ara-C, gemcitabine serves as a better transport substrate, is phosphorylated more efficiently, and is eliminated more slowly. These differences, together with self-potentiation, masked chain termination and the inhibition of ribonucleotide reductase, which are not seen with ara-C, may explain why gemcitabine is, and ara-C is not, active in solid tumours. This unique combination of metabolic properties and mechanistic characteristics suggests that gemcitabine is likely to be synergistic with other drugs that damage DNA, and also with other modalities such as radiation.
Evaluation of the antitumor activity of gemcitabine (2',2'-difluoro-2'-deoxycytidine).[Pubmed:2364394]
Cancer Res. 1990 Jul 15;50(14):4417-22.
A new pyrimidine antimetabolite, 2',2'-difluorodeoxycytidine, Gemcitabine (LY188011, dFdCyd) has been synthesized and evaluated in experimental tumor models. dFdCyd is a very potent and specific deoxycytidine analogue. The concentration required for 50% inhibition of growth is 1 ng/ml in the CCRF-CEM human leukemia cell culture assay. Concurrent addition of deoxycytidine to the cell culture system provides about a 1000-fold decrease in biological activity. The inhibition of growth of human leukemia cells in culture led to the in vivo evaluation of this compound as a potential oncolytic agent. Maximal activity in vivo was seen with dFdCyd when administered on an every third day schedule. 1-beta-D-Arabinofuranosylcytosine, administered on a daily for 10-day schedule, was directly compared to dFdCyd in this evaluation. dFdCyd demonstrated good to excellent antitumor activity in eight of the eight murine tumor models evaluated. 1-beta-D-Arabinofuranosylcytosine was substantially less active or had no activity in these same tumor models. This in vivo activity against murine solid tumors supports the conclusion that dFdCyd is an excellent candidate for clinical trials in the treatment of cancer.


