ML 202Pyruvate kinase M2 activator CAS# 1221186-52-2 |
- BMN-673 8R,9S
Catalog No.:BCC1422
CAS No.:1207456-00-5
- XAV-939
Catalog No.:BCC1120
CAS No.:284028-89-3
- PJ34
Catalog No.:BCC1865
CAS No.:344458-19-1
- ABT-888 (Veliparib)
Catalog No.:BCC1267
CAS No.:912444-00-9
- Veliparib dihydrochloride
Catalog No.:BCC2076
CAS No.:912445-05-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1221186-52-2 | SDF | Download SDF |
| PubChem ID | 44246498 | Appearance | Powder |
| Formula | C18H17N3O3S2 | M.Wt | 387.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
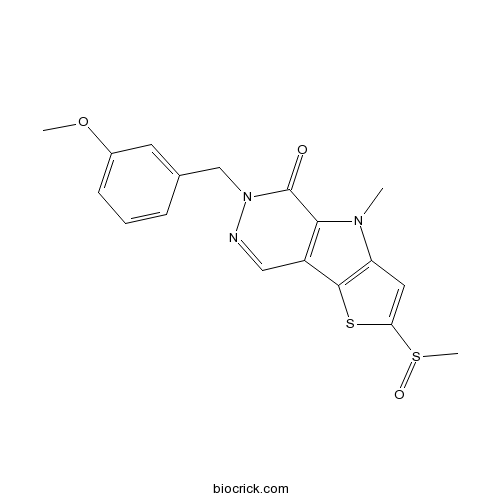
| Chemical Name | 6-[(3-methoxyphenyl)methyl]-4-methyl-2-methylsulfinylthieno[3,4]pyrrolo[1,3-d]pyridazin-5-one | ||
| SMILES | CN1C2=C(C3=C1C(=O)N(N=C3)CC4=CC(=CC=C4)OC)SC(=C2)S(=O)C | ||
| Standard InChIKey | MORBXZMIXGYQDB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H17N3O3S2/c1-20-14-8-15(26(3)23)25-17(14)13-9-19-21(18(22)16(13)20)10-11-5-4-6-12(7-11)24-2/h4-9H,10H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent activator of pyruvate kinase M2 (PKM2) (IC50 = 73 nM). Exhibits little or no activity against the pyruvate kinase isozymes PKM1, PKL and PKR. |

ML 202 Dilution Calculator

ML 202 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5808 mL | 12.9039 mL | 25.8078 mL | 51.6156 mL | 64.5195 mL |
| 5 mM | 0.5162 mL | 2.5808 mL | 5.1616 mL | 10.3231 mL | 12.9039 mL |
| 10 mM | 0.2581 mL | 1.2904 mL | 2.5808 mL | 5.1616 mL | 6.4519 mL |
| 50 mM | 0.0516 mL | 0.2581 mL | 0.5162 mL | 1.0323 mL | 1.2904 mL |
| 100 mM | 0.0258 mL | 0.129 mL | 0.2581 mL | 0.5162 mL | 0.6452 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dehydroborapetoside B
Catalog No.:BCN6601
CAS No.:1221178-16-0
- Acarbose sulfate
Catalog No.:BCC4284
CAS No.:1221158-13-9
- ER-878898
Catalog No.:BCC8958
CAS No.:122111-11-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- 2-Deoxy-2,2-difluoro-D-erythro-pentafuranous-1-ulose-3,5-dibenzoate
Catalog No.:BCC8575
CAS No.:122111-01-7
- Auraptenol
Catalog No.:BCN6113
CAS No.:1221-43-8
- 3,4-Dihydro-3,4-dihydroxynaphthalen-1(2H)-one
Catalog No.:BCN1602
CAS No.:1220891-22-4
- Charantadiol A
Catalog No.:BCN3483
CAS No.:1220890-23-2
- 2'-O-Acetylsprengerinin C
Catalog No.:BCN6655
CAS No.:1220707-33-4
- [bAla8]-Neurokinin A(4-10)
Catalog No.:BCC7137
CAS No.:122063-01-8
- Khayalenoid E
Catalog No.:BCN6111
CAS No.:1220508-29-1
- Monomethyl lithospermate B
Catalog No.:BCN2533
CAS No.:122021-74-3
- Meliasenin B
Catalog No.:BCN6112
CAS No.:1221262-77-6
- BP 554 maleate
Catalog No.:BCC6695
CAS No.:1221401-95-1
- Skepinone-L
Catalog No.:BCC1953
CAS No.:1221485-83-1
- Gymnemic acid I
Catalog No.:BCN2679
CAS No.:122168-40-5
- NPS-1034
Catalog No.:BCC6504
CAS No.:1221713-92-3
- 5'-Geranyl-5,7,2',4'-tetrahydroxyflavone
Catalog No.:BCN1601
CAS No.:1221762-70-4
- Boc-Dap(Fmoc)-OH
Catalog No.:BCC2665
CAS No.:122235-70-5
- 3-Epimeliasenin B
Catalog No.:BCN4723
CAS No.:1222475-77-5
- ML133 HCl
Catalog No.:BCC5006
CAS No.:1222781-70-5
- ML324
Catalog No.:BCC5575
CAS No.:1222800-79-4
- Torin 1
Catalog No.:BCC3676
CAS No.:1222998-36-8
- Torin 2
Catalog No.:BCC4606
CAS No.:1223001-51-1
miR-202 Diminishes TGFbeta Receptors and Attenuates TGFbeta1-Induced EMT in Pancreatic Cancer.[Pubmed:28373289]
Mol Cancer Res. 2017 Aug;15(8):1029-1039.
Previous studies in our laboratory identified that 3-deazaneplanocin A (DZNep), a carbocyclic adenosine analog and histone methyl transferase inhibitor, suppresses TGFbeta-induced epithelial-to-mesenchymal (EMT) characteristics. In addition, DZNep epigenetically reprograms miRNAs to regulate endogenous TGFbeta1 levels via miR-663/4787-mediated RNA interference (Mol Cancer Res. 2016 Sep 13. pii: molcanres.0083.2016) (1). Although DZNep also attenuates exogenous TGFbeta-induced EMT response, the mechanism of this inhibition was unclear. Here, DZNep induced miR-202-5p to target both TGFbeta receptors, TGFBR1 and TGFBR2, for RNA interference and thereby contributes to the suppression of exogenous TGFbeta-induced EMT in pancreatic cancer cells. Lentiviral overexpression of miR-202 significantly reduced the protein levels of both TGFbeta receptors and suppressed TGFbeta signaling and EMT phenotypic characteristics of cultured parenchymal pancreatic cancer cells. Consistently, transfection of anti-miRNAs against miR-202-5p resulted in increased TGFBR1 and TGFBR2 protein expressions and induced EMT characteristics in these cells. In stellate pancreatic cells, miR-202 overexpression slowed growth as well as reduced stromal extracellular membrane matrix protein expression. In orthotopic pancreatic cancer mouse models, both immunodeficient and immunocompetent, miR-202 reduced tumor burden and metastasis. Together, these findings demonstrate an alternative mechanism of DZNep in suppressing TGFbeta signaling at the receptor level and uncover the EMT-suppressing role of miR-202 in pancreatic cancer.Implications: These findings support the possibility of combining small molecule-based (e.g., DZNep analogs) or large molecule-based (e.g., miRNAs) epigenetic modifiers with conventional nucleoside analogs (e.g., gemcitabine, capecitabine) to improve the antimetastatic potential of current pancreatic cancer therapy. Mol Cancer Res; 15(8); 1029-39. (c)2017 AACR.
miR-202 Promotes Cell Apoptosis in Esophageal Squamous Cell Carcinoma by Targeting HSF2.[Pubmed:28277193]
Oncol Res. 2017 Jan 26;25(2):215-223.
Esophageal squamous cell carcinoma (ESCC) is one of the most common malignant cancers with high mortality around the world. However, the regulatory mechanism of ESCC carcinogenesis is not completely known. Here we demonstrate the novel role of miR-202 in regulating ESCC cell apoptosis. The analysis of data obtained from the GEO database showed that the expression of miR-202 is aberrantly decreased in tumor tissue from ESCC patients and cultured ESCC cell lines. After transfection with miR-202 mimic or inhibitor, the apoptotic capacity of ESCC cells was significantly increased by miR-202 overexpression but reduced by miR-202 repression. We then identified HSF2 as a direct target of miR-202 with the binding site on the 3'-UTR of HSF2 mRNA in ESCC cells. The apoptosis of ESCC cells induced by the miR-202 mimic could be repressed by HSF2 overexpression. Further studies indicated that HSF2 overexpression strongly upregulated the expression of Hsp70 at both the mRNA and protein levels. In addition, HSF2/Hsp70 suppressed ESCC cell apoptosis by preventing caspase 3 activation. In conclusion, miR-202 is a potential tumor suppressor in human ESCC and acts by regulating the apoptosis of ESCC cells by targeting HSF2, in which caspase 3 activation is involved. This might provide a novel therapeutic target for human ESCC.
Discovery of (R)-1-(3-(4-Amino-3-(3-chloro-4-(pyridin-2-ylmethoxy)phenyl)-1H-pyrazolo[3,4-d]py rimidin-1-yl)piperidin-1-yl)prop-2-en-1-one (CHMFL-EGFR-202) as a Novel Irreversible EGFR Mutant Kinase Inhibitor with a Distinct Binding Mode.[Pubmed:28282122]
J Med Chem. 2017 Apr 13;60(7):2944-2962.
On the basis of Ibrutinib's core pharmacophore, which was moderately active to EGFR T790M mutant, we discovered novel epidermal growth factor receptor (EGFR) inhibitor compound 19 (CHMFL-EGFR-202), which potently inhibited EGFR primary mutants (L858R, del19) and drug-resistant mutant L858R/T790M. Compound 19 displayed a good selectivity profile among 468 kinases/mutants tested in the KINOMEscan assay (S score (1) = 0.02). In particular, it did not exhibit apparent activities against INSR and IGF1R kinases. The X-ray crystal structure revealed that this class of inhibitors formed a covalent bond with Cys797 in a distinct "DFG-in-C-helix-out" inactive EGFR conformation. Compound 19 displayed strong antiproliferative effects against EGFR mutant-driven nonsmall cell lung cancer (NSCLC) cell lines such as H1975, PC9, HCC827, and H3255 but not the wild-type EGFR expressing cells. In the H1975 and PC9 cell-inoculated xenograft mouse models, compound 19 exhibited dose-dependent tumor growth suppression efficacy without obvious toxicity. Compound 19 might be a potential drug candidate for EGFR mutant-driven NSCLC.
Evaluation of thieno[3,2-b]pyrrole[3,2-d]pyridazinones as activators of the tumor cell specific M2 isoform of pyruvate kinase.[Pubmed:20451379]
Bioorg Med Chem Lett. 2010 Jun 1;20(11):3387-93.
Cancer cells have distinct metabolic needs that are different from normal cells and can be exploited for development of anti-cancer therapeutics. Activation of the tumor specific M2 form of pyruvate kinase (PKM2) is a potential strategy for returning cancer cells to a metabolic state characteristic of normal cells. Here, we describe activators of PKM2 based upon a substituted thieno[3,2-b]pyrrole[3,2-d]pyridazinone scaffold. The synthesis of these agents, structure-activity relationships, analysis of activity at related targets (PKM1, PKR and PKL) and examination of aqueous solubility are investigated. These agents represent the second reported chemotype for activation of PKM2.


