ABT-888 (Veliparib)Potent PARP inhibitor CAS# 912444-00-9 |
- BYK 204165
Catalog No.:BCC2449
CAS No.:1104546-89-5
- EB 47
Catalog No.:BCC2452
CAS No.:1190332-25-2
- BYK 49187
Catalog No.:BCC2450
CAS No.:163120-31-8
- A-966492
Catalog No.:BCC2211
CAS No.:934162-61-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 912444-00-9 | SDF | Download SDF |
| PubChem ID | 11960529 | Appearance | Powder |
| Formula | C13H16N4O | M.Wt | 244.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ABT-888 | ||
| Solubility | DMSO : ≥ 29 mg/mL (118.71 mM) *"≥" means soluble, but saturation unknown. | ||
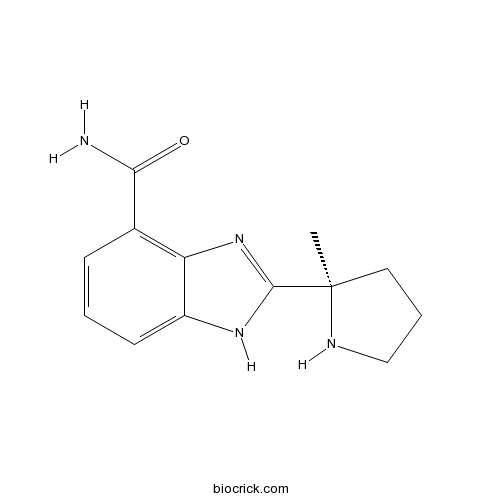
| Chemical Name | 2-[(2R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide | ||
| SMILES | CC1(CCCN1)C2=NC3=C(C=CC=C3N2)C(=O)N | ||
| Standard InChIKey | JNAHVYVRKWKWKQ-CYBMUJFWSA-N | ||
| Standard InChI | InChI=1S/C13H16N4O/c1-13(6-3-7-15-13)12-16-9-5-2-4-8(11(14)18)10(9)17-12/h2,4-5,15H,3,6-7H2,1H3,(H2,14,18)(H,16,17)/t13-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Veliparib (ABT-888) is a potent inhibitor of PARP1 and PARP2 with Ki of 5.2 nM and 2.9 nM, respectively. | |||||
| Targets | PARP1 | PARP2 | ||||
| IC50 | 5.2 nM (Ki) | 2.9 nM (Ki) | ||||
| Cell experiment: | |
| Cell lines | HCT-116 and HT-29 cell lines |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 4 μM; 24 h |
| Applications | In HCT-116 and HT-29 cell lines, the ability of ABT-888 to synergize the effect of the anti-cancer agents, SN38 or oxaliplatin, was determined using the SRB assay. PARP activity was significantly reduced in samples treated with SN38 in combination with ABT-888 (>4 fold at 24 h). |
| Animal experiment: | |
| Animal models | Female nude athymic mice |
| Dosage form | 12.5 mg/kg; oral gavage twice daily in 6-hour intervals. |
| Application | HCT116 xenografts were established in 5- to 6-week-old female nude athymic mice by subcutaneous flank injections of 200 mL cell suspension (5*106cells) per flank. This triple-therapy group (RT, CPT-11, and ABT) showed a significantly longer tumor growth delay (TGD) when compared with the tumors treated with RT and CPT-11 but no ABT-888, which had a mean TGD of 14.21 days. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Davidson D, Wang Y, Aloyz R, et al. The PARP inhibitor ABT-888 synergizes irinotecan treatment of colon cancer cell lines[J]. Investigational new drugs, 2013, 31(2): 461-468. [2] Shelton J W, Waxweiler T V, Landry J, et al. In vitro and in vivo enhancement of chemoradiation using the oral parp inhibitor ABT-888 in colorectal cancer cells[J]. International Journal of Radiation Oncology* Biology* Physics, 2013, 86(3): 469-476. | |

ABT-888 (Veliparib) Dilution Calculator

ABT-888 (Veliparib) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0933 mL | 20.4666 mL | 40.9333 mL | 81.8666 mL | 102.3332 mL |
| 5 mM | 0.8187 mL | 4.0933 mL | 8.1867 mL | 16.3733 mL | 20.4666 mL |
| 10 mM | 0.4093 mL | 2.0467 mL | 4.0933 mL | 8.1867 mL | 10.2333 mL |
| 50 mM | 0.0819 mL | 0.4093 mL | 0.8187 mL | 1.6373 mL | 2.0467 mL |
| 100 mM | 0.0409 mL | 0.2047 mL | 0.4093 mL | 0.8187 mL | 1.0233 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
ABT-888 is an orally available inhibitor of poly(ADP-ribose) polymerase.
Abstract
Treatments of ABT-888 (0.125 μΜ) with irinotecan and ABT-888 (0.5-4 μM) both exhibited synergistic effect against colon cancer cell lines, in which the synergy of ABT-888/irinotecan is correlated with the degree of PARP1 inhibition and results in increased G2/M cell cycle arrest and increased DNA damage after 24 h and increased apoptosis after 48 h.
Abstract
ABT-888 is a potent inhibitor of both PARP1 and PARP2.
Abstract
ABT-888/carboplatin treatment exhibits a stronger ability to kill or inhibit proliferation of Brca/BRCA-deficient cells than cisplatin or carboplatin alone, which delayed tumor growth in Brca2 xenografts and induced DNA damage and apoptosis.
Abstract
The combination of ABT-888 (10 mg) and topotecan (0.6 mg/m2/d) was orally administered to enrolled patients twice a day for the first 5 days during a 21-day cycle, in which a >75% reduction in PAR levels, a >50% reduction in PBMCs and increased γH2AX response in CTC and PBMCs were observed in patients.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
ABT-888, also named as Veliparib, is poly (ADP-ribose) polymerase (PARP) inhibitor and has demonstrated excellent in vivo efficacy in a broad spectrum of preclinical tumor models in combination with a variety of cytotoxic agents. PARP is involved in DNA repair and elevated PARP levels can result in resistance to cytotoxic chemotherapy and radiation. So, PARP inhibitors hold promise as chemotherapy and radiation sensitizers. ABT-888 is also active on microsatellite instability (MSI) cell lines harboring mutations in both MRE11 and RAD50 genes compared to microsatellite stable (MSS) cell lines (wild-type for both genes).
Reference
Shivaani Kummar, Robert Kinders, Martin E. Gutierrez, Larry Rubinstein, Ralph E. Parchment, Lawrence R. Phillips, Jiuping Ji, Anne Monks, Jennifer A. Low, Alice Chen, Anthony J. Murgo, Jerry Collins, Seth M. Steinberg, Helen Eliopoulos, Vincent L. Giranda, Gary Gordon, Lee Helman, Robert Wiltrout, Joseph E. Tomaszewski and James H. Doroshow. Phase 0 Clinical Trial of the Poly (ADP-Ribose) Polymerase Inhibitor ABT-888 in Patients With Advanced Malignancies. Journal of Clinical Oncology. 2009; 27(16): 2705 – 11.
Xiaofeng Li, Juergen Delzer, Richard Voorman, Sonia M. de Morais and Yanbin Lao. Disposition and Drug-Drug Interaction Potential of Veliparib (ABT-888), a Novel and Potent Inhibitor of Poly (ADP-ribose) Polymerase. DRUG METABOLISM AND DISPOSITION. 2011; 39(7): 1161 – 69.
E. Vilar Sanchez, A. Chow, L. Raskin, M. D. Iniesta, B. Mukherjee and S. B. Gruber. Preclinical testing of the PARP inhibitor ABT-888 in microsatellite instable colorectal cancer. Journal of Clinical Oncology. 2009; 27(15S): 11028A.
- TCB-2
Catalog No.:BCC7421
CAS No.:912342-28-0
- Karavilagenin A
Catalog No.:BCN4455
CAS No.:912329-03-4
- Spantide I
Catalog No.:BCC5808
CAS No.:91224-37-2
- Noscapine HCl
Catalog No.:BCC3819
CAS No.:912-60-7
- H-Met-OtBu.HCl
Catalog No.:BCC2996
CAS No.:91183-71-0
- Ophiopogonin C
Catalog No.:BCN5379
CAS No.:911819-08-4
- Lucyoside B
Catalog No.:BCN7811
CAS No.:91174-19-5
- Chrysothol
Catalog No.:BCN4454
CAS No.:911714-91-5
- 17-AAG Hydrochloride
Catalog No.:BCC1297
CAS No.:911710-03-7
- Terbinafine
Catalog No.:BCC3865
CAS No.:91161-71-6
- Infractin
Catalog No.:BCN3652
CAS No.:91147-07-8
- SLx-2119
Catalog No.:BCC1954
CAS No.:911417-87-3
- Veliparib dihydrochloride
Catalog No.:BCC2076
CAS No.:912445-05-7
- SAG
Catalog No.:BCC6390
CAS No.:912545-86-9
- Melilotigenin B
Catalog No.:BCN4456
CAS No.:91269-84-0
- Sarafloxacin HCl
Catalog No.:BCC4713
CAS No.:91296-87-6
- AT13387
Catalog No.:BCC2122
CAS No.:912999-49-6
- ELN441958
Catalog No.:BCC6452
CAS No.:913064-47-8
- LY 2087101
Catalog No.:BCC7869
CAS No.:913186-74-0
- Almorexant hydrochloride
Catalog No.:BCC5123
CAS No.:913358-93-7
- AMG-458
Catalog No.:BCC3721
CAS No.:913376-83-7
- Brexpiprazole
Catalog No.:BCC4118
CAS No.:913611-97-9
- 1''-Hydroxyerythrinin C
Catalog No.:BCN4066
CAS No.:913690-46-7
- Ropinirole HCl
Catalog No.:BCC4939
CAS No.:91374-20-8
Phase I Safety, Pharmacokinetic, and Pharmacodynamic Study of the Poly(ADP-ribose) Polymerase (PARP) Inhibitor Veliparib (ABT-888) in Combination with Irinotecan in Patients with Advanced Solid Tumors.[Pubmed:26842236]
Clin Cancer Res. 2016 Jul 1;22(13):3227-37.
PURPOSE: PARP is essential for recognition and repair of DNA damage. In preclinical models, PARP inhibitors modulate topoisomerase I inhibitor-mediated DNA damage. This phase I study determined the MTD, dose-limiting toxicities (DLT), pharmacokinetics (PK), and pharmacodynamics (PD) of veliparib, an orally bioavailable PARP1/2 inhibitor, in combination with irinotecan. EXPERIMENTAL DESIGN: Patients with advanced solid tumors were treated with 100 mg/m(2) irinotecan on days 1 and 8 of a 21-day cycle. Twice-daily oral dosing of veliparib (10-50 mg) occurred on days 3 to 14 (cycle 1) and days -1 to 14 (subsequent cycles) followed by a 6-day rest. PK studies were conducted with both agents alone and in combination. Paired tumor biopsies were obtained after irinotecan alone and veliparib/irinotecan to evaluate PARP1/2 inhibition and explore DNA damage signals (nuclear gamma-H2AX and pNBS1). RESULTS: Thirty-five patients were treated. DLTs included fatigue, diarrhea, febrile neutropenia, and neutropenia. The MTD was 100 mg/m(2) irinotecan (days 1 and 8) combined with veliparib 40 mg twice daily (days -1-14) on a 21-day cycle. Of 31 response-evaluable patients, there were six (19%) partial responses. Veliparib exhibited linear PK, and there were no apparent PK interactions between veliparib and irinotecan. At all dose levels, veliparib reduced tumor poly(ADP-ribose) (PAR) content in the presence of irinotecan. Several samples showed increases in gamma-H2AX and pNBS1 after veliparib/irinotecan compared with irinotecan alone. CONCLUSIONS: Veliparib can be safely combined with irinotecan at doses that inhibit PARP catalytic activity. Preliminary antitumor activity justifies further evaluation of the combination. Clin Cancer Res; 22(13); 3227-37. (c)2016 AACR.
Development of a Level A in Vitro-in Vivo Correlation for Veliparib (ABT-888) Extended Release Tablet Formulation.[Pubmed:28243955]
Pharm Res. 2017 Jun;34(6):1187-1192.
PURPOSE: The aim of the current manuscript is to develop and validate a level A in vitro-in vivo correlation (IVIVC) for veliparib extended-release (ER) tablet formulations. METHODS: The in vitro release profiles of veliparib formulations were determined using USP Dissolution Apparatus 2 with 900 mL of 0.1 N HCl at 75 rpm. In a clinical study, 24 subjects with solid tumors received one of the ER formulations (200 mg): fast (Formulation A), intermediate (Formulation B), and slow (Formulation C), and two 100 mg immediate release capsules (Formulation D). Blood samples were collected over a period of 48 h and analyzed using LCMS/MS. A linear correlation model was developed using fraction absorbed and fraction dissolved data from formulations A and B. Besides assessing internal predictability, external predictability was evaluated using formation C. Prediction errors were estimated for maximum observed plasma concentration (Cmax) and area under the plasma-concentration time curve from zero to last measured time point (AUCt) to determine the predictive ability of the correlation. RESULTS: There was a significant linear relationship (r(2) = 0.944) between the fraction of drug absorbed and the fraction of drug dissolved. The prediction error using the internal validation for Cmax and AUCt were below 15% for the individual formulations and below 10% for the average. The prediction error in AUCt and Cmax for formulation C was 5% and 11%, respectively. CONCLUSIONS: A level A IVIVC for the veliparib ER tablet formulation was established. The IVIVC may allow the associated dissolution data to be used as a surrogate for bioavailability.
Effect of veliparib (ABT-888) on cardiac repolarization in patients with advanced solid tumors: a randomized, placebo-controlled crossover study.[Pubmed:27709282]
Cancer Chemother Pharmacol. 2016 Nov;78(5):1003-1011.
PURPOSE: Veliparib (ABT-888) is an orally bioavailable potent inhibitor of poly(ADP-ribose) polymerase (PARP)-1 and PARP-2. This phase 1 study evaluated the effect of veliparib on corrected QT interval using Fridericia's formula (QTcF). METHODS: Eligible patients with advanced solid tumors received single-dose oral veliparib (200 mg or 400 mg) or placebo in a 6-sequence, 3-period crossover design. The primary endpoint was the difference in the mean baseline-adjusted QTcF between 400 mg veliparib and placebo (QTcF) at six post-dose time points. Absence of clinically relevant QTcF effect was shown if the 95 % upper confidence bound (UCB) for the mean QTcF was <10 ms for all time points. An exposure-response analysis was also performed. RESULTS: Forty-seven patients were enrolled. Maximum mean QTcF of veliparib 400 mg was 6.4 ms, with a 95 % UCB of 8.9 ms; for veliparib 200 mg, the maximum mean QTcF was 3.6 ms, with a 95 % UCB of 6.1 ms. No patient had a QTcF value >480 ms or change from baseline in QTcF interval >30 ms. Treatment-emergent adverse events (TEAEs) were experienced by 36.2, 48.9, and 47.8 % of patients while receiving veliparib 200 mg, veliparib 400 mg, and placebo, respectively. Most common TEAEs were nausea (12.8 %) and myalgia (8.5 %) after veliparib 200 mg, nausea (8.5 %) and vomiting (8.5 %) after veliparib 400 mg, and nausea (6.5 %) after placebo. CONCLUSIONS: Single-dose veliparib (200 mg or 400 mg) did not result in clinically significant QTc prolongation and was well tolerated in patients with advanced solid tumors.
A final report of a phase I study of veliparib (ABT-888) in combination with low-dose fractionated whole abdominal radiation therapy (LDFWAR) in patients with advanced solid malignancies and peritoneal carcinomatosis with a dose escalation in ovarian and fallopian tube cancers.[Pubmed:28109627]
Gynecol Oncol. 2017 Mar;144(3):486-490.
BACKGROUND: The combination of low-dose radiation therapy with PARP inhibition enhances anti-tumor efficacy through potentiating DNA damage. We combined low-dose fractionated whole abdominal radiation (LDFWAR) with ABT-888 in patients with peritoneal carcinomatosis with a dose escalation in ovarian and fallopian cancer patients (OV). METHODS: Patients were treated with veliparib, 40-400mg orally BID on days 1-21 of 3 28-day cycles on 6 dose levels. Dose levels 5 and 6 included only OV patients. LDFWAR consisted of 21.6Gy in 36 fractions, 0.6Gy twice daily on days 1 and 5 for weeks 1-3 of each cycle. Circulating tumor material and quality of life were serially assessed. RESULTS: 32pts were treated. Median follow-up was 45months (10-50). The most common treatment-related grade 3 and 4 toxicities were lymphopenia (59%), anemia (9%), thrombocytopenia (12%), neutropenia (6%), leukopenia (6%), nausea (6%), diarrhea (6%), anorexia (6%), vomiting (6%) and fatigue (6%). The maximum tolerated dose was determined to be 250mg PO BID. Median PFS was 3.6months and median OS was 9.1months. In OV patients, OS was longer for platinum-sensitive patients (10.9mo) compared to platinum-resistant patients (5.8mo). QoL decreased for all groups during treatment. Germline BRCA status was known for 14/18 patients with OV cancers, 5 of whom were BRCA mutation carriers. One objective response (3%) was observed. CONCLUSION: ABT-888 plus LDFWAR is tolerable with gastrointestinal symptoms, fatigue and myelosuppression as the most common toxicities. The single observed objective response was in a germline BRCA mutated, platinum-sensitive patient.


