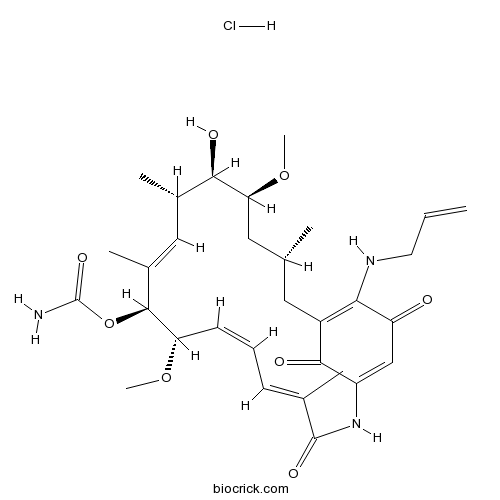
17-AAG HydrochlorideHsp90 inhibitor,geldanamycin analogue CAS# 911710-03-7 |
- Scrambled 10Panx
Catalog No.:BCC1246
CAS No.:1315378-72-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 911710-03-7 | SDF | Download SDF |
| PubChem ID | 66576986 | Appearance | Powder |
| Formula | C31H44ClN3O8 | M.Wt | 622.15 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Tanespimycin Hydrochloride; NSC 330507 Hydrochloride; CP 127374 Hydrochloride | ||
| Solubility | Soluble in DMSO | ||
| Chemical Name | [(3R,5S,6R,7S,8E,10S,11S,12Z,14E)-6-hydroxy-5,11-dimethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-21-(prop-2-enylamino)-17-azabicyclo[16.3.1]docosa-1(21),8,12,14,18-pentaen-10-yl] carbamate;hydrochloride | ||
| SMILES | CC1CC(C(C(C=C(C(C(C=CC=C(C(=O)NC2=CC(=O)C(=C(C1)C2=O)NCC=C)C)OC)OC(=O)N)C)C)O)OC.Cl | ||
| Standard InChIKey | VFOPOIJGNBNWPN-AFXVCOSJSA-N | ||
| Standard InChI | InChI=1S/C31H43N3O8.ClH/c1-8-12-33-26-21-13-17(2)14-25(41-7)27(36)19(4)15-20(5)29(42-31(32)39)24(40-6)11-9-10-18(3)30(38)34-22(28(21)37)16-23(26)35;/h8-11,15-17,19,24-25,27,29,33,36H,1,12-14H2,2-7H3,(H2,32,39)(H,34,38);1H/b11-9-,18-10+,20-15+;/t17-,19+,24+,25+,27-,29+;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 17-AAG Hydrochloride is a potent HSP90 inhibitor with IC50 of 5 nM, having a 100-fold higher binding affinity for HSP90 derived from tumour cells than HSP90 from normal cells.In Vitro:17-AAG causes the degradation of HER2, Akt, and both mutant and wild-type AR and the retinoblastoma-dependent G1 growth arrest of prostate cancer cells. 17-AAG inhibits prostate cancer cell lines with IC50s ranged from 25-45 nM (LNCaP, 25 nM; LAPC-4, 40 nM; DU-145, 45 nM; and PC-3, 25 nM)[1]. Combination of 17-AAG (10 nM) and Trastuzumab induces more effective ErbB2-degradation. 17-AAG (0.1-1 μM) induces a nearly complete loss of ErbB2 on ErbB2-overexpressing breast cancer cells[2]. 17-AAG inhibits cell growth and induces G2/M cell cycle arrest and apoptosis in CCA cells together with the down-regulation of Bcl-2, Survivin and Cyclin B1, and the up-regulation of cleaved PARP[3].In Vivo:17-AAG (25-200 mg/kg, i.p.) causes a dose-dependent decline in AR, HER2, and Akt expression in prostate cancer xenografts. 17-AAG treatment at doses sufficient to induce AR, HER2, and Akt degradation results in the dose-dependent inhibition of androgen-dependent and -independent prostate cancer xenograft growth without toxicity[1]. 17-AAG (60 mg/kg) with paclitaxel (60 mg/kg) and rapamycin (30 mg/kg) inhibits A549 and MDA-MB-231 tumor growth far more potently than paclitaxel-containing micelles and effected tumor cures in MDA-MB-231 tumor-bearing animals by tail vein injection[4]. References: | |||||

17-AAG Hydrochloride Dilution Calculator

17-AAG Hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6073 mL | 8.0366 mL | 16.0733 mL | 32.1466 mL | 40.1832 mL |
| 5 mM | 0.3215 mL | 1.6073 mL | 3.2147 mL | 6.4293 mL | 8.0366 mL |
| 10 mM | 0.1607 mL | 0.8037 mL | 1.6073 mL | 3.2147 mL | 4.0183 mL |
| 50 mM | 0.0321 mL | 0.1607 mL | 0.3215 mL | 0.6429 mL | 0.8037 mL |
| 100 mM | 0.0161 mL | 0.0804 mL | 0.1607 mL | 0.3215 mL | 0.4018 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
17-AAG is a less toxic analogue of the geldanamycin which binds to Hsp90 and alters its function.
- Terbinafine
Catalog No.:BCC3865
CAS No.:91161-71-6
- Infractin
Catalog No.:BCN3652
CAS No.:91147-07-8
- SLx-2119
Catalog No.:BCC1954
CAS No.:911417-87-3
- EC 144
Catalog No.:BCC5600
CAS No.:911397-80-3
- MPP dihydrochloride
Catalog No.:BCC7225
CAS No.:911295-24-4
- LY2603618
Catalog No.:BCC3923
CAS No.:911222-45-2
- Cefoselis hydrochloride
Catalog No.:BCC4093
CAS No.:911212-25-4
- Adapalene sodium salt
Catalog No.:BCC4285
CAS No.:911110-93-5
- SB 706504
Catalog No.:BCC5615
CAS No.:911110-38-8
- Isotussilagine
Catalog No.:BCN1985
CAS No.:91108-32-6
- Furowanin A
Catalog No.:BCN4790
CAS No.:911004-72-3
- 3,6,19,23-Tetrahydroxy-12-ursen-28-oic acid
Catalog No.:BCN1310
CAS No.:91095-51-1
- Chrysothol
Catalog No.:BCN4454
CAS No.:911714-91-5
- Lucyoside B
Catalog No.:BCN7811
CAS No.:91174-19-5
- Ophiopogonin C
Catalog No.:BCN5379
CAS No.:911819-08-4
- H-Met-OtBu.HCl
Catalog No.:BCC2996
CAS No.:91183-71-0
- Noscapine HCl
Catalog No.:BCC3819
CAS No.:912-60-7
- Spantide I
Catalog No.:BCC5808
CAS No.:91224-37-2
- Karavilagenin A
Catalog No.:BCN4455
CAS No.:912329-03-4
- TCB-2
Catalog No.:BCC7421
CAS No.:912342-28-0
- ABT-888 (Veliparib)
Catalog No.:BCC1267
CAS No.:912444-00-9
- Veliparib dihydrochloride
Catalog No.:BCC2076
CAS No.:912445-05-7
- SAG
Catalog No.:BCC6390
CAS No.:912545-86-9
- Melilotigenin B
Catalog No.:BCN4456
CAS No.:91269-84-0
A phase 1 study of IPI-504 (retaspimycin hydrochloride) in patients with relapsed or relapsed and refractory multiple myeloma.[Pubmed:21851215]
Leuk Lymphoma. 2011 Dec;52(12):2308-15.
Abstract A phase 1 study of IPI-504 (retaspimycin hydrochloride) administered intravenously twice weekly for 2 weeks at 22.5, 45, 90, 150, 225, 300 or 400 mg/m(2) followed by 10 days off-treatment was conducted to determine the safety and maximum tolerated dose (MTD) of IPI-504 in patients with relapsed or relapsed/refractory multiple myeloma (MM). Anti-tumor activity and pharmacokinetics were also evaluated. Eighteen patients (mean age 60.5 years; median 9 prior therapies) were enrolled. No dose-limiting toxicities (DLTs) were reported for IPI-504 doses up to 400 mg/m(2). The most common treatment-related adverse event was grade 1 infusion site pain (four patients). All other treatment-related events were assessed as grade 1 or 2 in severity. The area under the curve (AUC) increased with increasing dose, and the mean half-life was approximately 2-4 h for IPI-504 and its metabolites. Four patients had stable disease, demonstrating modest single-agent activity in relapsed or relapsed/refractory MM.
Geldanamycin and its derivatives as Hsp90 inhibitors.[Pubmed:22652777]
Front Biosci (Landmark Ed). 2012 Jun 1;17:2269-77.
The Hsp90 molecule, one of the most abundant heat shock proteins in mammalian cells, maintains homeostasis and prevents stress-induced cellular damage. Hsp90 is expressed under normal conditions at a level of about 1-2 Percent of total proteins, while its expression increases 2-10 fold in cancer cells. The two main constitutively expressed isoforms of Hsp90 are known as Hsp90-alpha and Hsp90-beta, and their upregulation is associated with tumor progression, invasion and formation of metastases, as well as development of drug resistance. The Hsp90 is a key target for many newly established, potent anticancer agents containing Hsp90 N-terminal ATP binding inhibitors, such as geldanamycin, and its analogues 17AAG and 17DMAG. The therapeutic usage of geldanamycin has been limited due to its poor water solubility and severe hepatotoxicity. Therefore, its analogues, including 17AAG, 17DMAG, Tanespimycin and Retaspimycin hydrochloride, with improved pharmacokinetic profiles, have been developed.
The heat shock protein 90 inhibitor IPI-504 induces KIT degradation, tumor shrinkage, and cell proliferation arrest in xenograft models of gastrointestinal stromal tumors.[Pubmed:21825009]
Mol Cancer Ther. 2011 Oct;10(10):1897-908.
The activity of the receptor tyrosine kinase KIT is crucial for gastrointestinal stromal tumor (GIST) growth and survival. Imatinib and sunitinib are very effective in advanced GIST, but have no curative potential. The observation that heat shock protein 90 (HSP90) inhibition results in KIT degradation prompted us to assess the efficacy of the HSP90 inhibitor retaspimycin hydrochloride (IPI-504) alone or in combination with imatinib or sunitinib in two GIST xenografts with distinctive KIT mutations. Nude mice were grafted with human GIST carrying KIT exon 13 (GIST-882; n = 59) or exon 11 (GIST-PSW; n = 44) mutations and dosed with imatinib (50 mg/kg twice daily), sunitinib (40 mg/kg once daily), IPI-504 (100 mg/kg 3 times per week), IPI-504 + imatinib, or IPI-504 + sunitinib. We evaluated tumor volume, proliferation and apoptosis, KIT expression and activation, as well as adverse events during treatment. Treatment with IPI-504 alone resulted in tumor regression, proliferation arrest, and induction of tumor necrosis. We documented downregulation of KIT and its signaling cascade in IPI-504-treated animals. Treatment effects were enhanced by combining IPI-504 with imatinib or sunitinib. On histologic examination, liver damage was frequently observed in animals exposed to combination treatments. In conclusion, IPI-504 shows consistent antitumor activity and induces KIT downregulation in GIST, as a single agent, and is more potent in combination with imatinib or sunitinib. The sequence of drug administration in the combination arms warrants further studies.
A phase I study of the HSP90 inhibitor retaspimycin hydrochloride (IPI-504) in patients with gastrointestinal stromal tumors or soft-tissue sarcomas.[Pubmed:24045182]
Clin Cancer Res. 2013 Nov 1;19(21):6020-9.
PURPOSE: Heat shock protein 90 (HSP90) is required for the proper folding, function, and stability of various client proteins, two of which (KIT and PDGFRalpha) are critical in the pathogenesis and progression of gastrointestinal stromal tumors (GIST). This phase I study investigated the safety and maximum tolerated dose (MTD) of retaspimycin hydrochloride (IPI-504), a novel potent and selective HSP90 inhibitor, in patients with metastatic and/or unresectable GIST or other soft-tissue sarcomas (STS). EXPERIMENTAL DESIGN: IPI-504 was administered intravenously at doses ranging from 90 to 500 mg/m(2) twice weekly for 2 weeks on/1 week off. Safety, pharmacokinetic, and pharmacodynamic profiles were determined. Response was assessed by Response Evaluation Criteria for Solid Tumors (RECIST) 1.0 and optionally via 18-fluorodeoxyglucose positron emission tomography (18-FDG-PET) imaging. RESULTS: Fifty-four patients received IPI-504; 37 with GIST and 17 with other STS. The MTD was 400 mg/m(2) twice weekly for 2 weeks on/1 week off. Common related adverse events were fatigue (59%), headache (44%), and nausea (43%). Exposure to IPI-504, 17-AAG, and 17-AG increased with IPI-504 dose. Stable disease (SD) was observed in 70% (26 of 37) of patients with GIST and 59% (10 of 17) of patients with STS. There was one confirmed partial response (PR) in a patient with GIST and one PR in a patient with liposarcoma. Metabolic partial responses occurred in 11 of 29 (38%) patients with GIST. CONCLUSIONS: In this study of advanced GIST or other STS, IPI-504 was generally well-tolerated with some evidence of antitumor activity, serving as a clinical proof-of-concept that HSP90 inhibition remains a promising strategy.


