MPP dihydrochlorideCAS# 911295-24-4 |
- MTEP hydrochloride
Catalog No.:BCC1780
CAS No.:1186195-60-7
- mGlu2 agonist
Catalog No.:BCC1745
CAS No.:1311385-32-6
- LY341495
Catalog No.:BCC1724
CAS No.:201943-63-7
- CPPHA
Catalog No.:BCC1501
CAS No.:693288-97-0
- Dipraglurant
Catalog No.:BCC1531
CAS No.:872363-17-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 911295-24-4 | SDF | Download SDF |
| PubChem ID | 45073474 | Appearance | Powder |
| Formula | C29H31N3O3.2HCl | M.Wt | 542.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
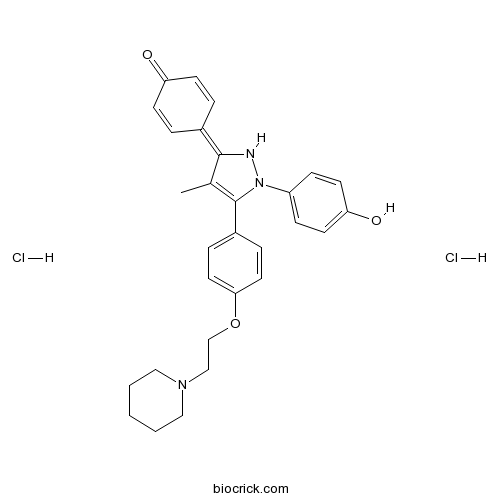
| Chemical Name | 4-[2-(4-hydroxyphenyl)-4-methyl-3-[4-(2-piperidin-1-ylethoxy)phenyl]-1H-pyrazol-5-ylidene]cyclohexa-2,5-dien-1-one;dihydrochloride | ||
| SMILES | CC1=C(N(NC1=C2C=CC(=O)C=C2)C3=CC=C(C=C3)O)C4=CC=C(C=C4)OCCN5CCCCC5.Cl.Cl | ||
| Standard InChIKey | SMPRQALBFACGGO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H31N3O3.2ClH/c1-21-28(22-5-11-25(33)12-6-22)30-32(24-9-13-26(34)14-10-24)29(21)23-7-15-27(16-8-23)35-20-19-31-17-3-2-4-18-31;;/h5-16,30,34H,2-4,17-20H2,1H3;2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective, high affinity silent antagonist at ERα receptors. Displays > 200-fold selectivity for ERα over ERβ. Ki values are 2.7 and 1800 nM at ERα and ERβ receptors respectively. |

MPP dihydrochloride Dilution Calculator

MPP dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8433 mL | 9.2166 mL | 18.4332 mL | 36.8664 mL | 46.0829 mL |
| 5 mM | 0.3687 mL | 1.8433 mL | 3.6866 mL | 7.3733 mL | 9.2166 mL |
| 10 mM | 0.1843 mL | 0.9217 mL | 1.8433 mL | 3.6866 mL | 4.6083 mL |
| 50 mM | 0.0369 mL | 0.1843 mL | 0.3687 mL | 0.7373 mL | 0.9217 mL |
| 100 mM | 0.0184 mL | 0.0922 mL | 0.1843 mL | 0.3687 mL | 0.4608 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- LY2603618
Catalog No.:BCC3923
CAS No.:911222-45-2
- Cefoselis hydrochloride
Catalog No.:BCC4093
CAS No.:911212-25-4
- Adapalene sodium salt
Catalog No.:BCC4285
CAS No.:911110-93-5
- SB 706504
Catalog No.:BCC5615
CAS No.:911110-38-8
- Isotussilagine
Catalog No.:BCN1985
CAS No.:91108-32-6
- Furowanin A
Catalog No.:BCN4790
CAS No.:911004-72-3
- 3,6,19,23-Tetrahydroxy-12-ursen-28-oic acid
Catalog No.:BCN1310
CAS No.:91095-51-1
- 8-Epidiosbulbin E acetate
Catalog No.:BCN7812
CAS No.:91095-48-6
- Danshenol C
Catalog No.:BCN6681
CAS No.:910856-25-6
- BAY 60-6583
Catalog No.:BCC6197
CAS No.:910487-58-0
- RGDS peptide
Catalog No.:BCC7694
CAS No.:91037-65-9
- CGI-1746
Catalog No.:BCC1473
CAS No.:910232-84-7
- EC 144
Catalog No.:BCC5600
CAS No.:911397-80-3
- SLx-2119
Catalog No.:BCC1954
CAS No.:911417-87-3
- Infractin
Catalog No.:BCN3652
CAS No.:91147-07-8
- Terbinafine
Catalog No.:BCC3865
CAS No.:91161-71-6
- 17-AAG Hydrochloride
Catalog No.:BCC1297
CAS No.:911710-03-7
- Chrysothol
Catalog No.:BCN4454
CAS No.:911714-91-5
- Lucyoside B
Catalog No.:BCN7811
CAS No.:91174-19-5
- Ophiopogonin C
Catalog No.:BCN5379
CAS No.:911819-08-4
- H-Met-OtBu.HCl
Catalog No.:BCC2996
CAS No.:91183-71-0
- Noscapine HCl
Catalog No.:BCC3819
CAS No.:912-60-7
- Spantide I
Catalog No.:BCC5808
CAS No.:91224-37-2
- Karavilagenin A
Catalog No.:BCN4455
CAS No.:912329-03-4
Effects of low-magnitude high-frequency vibration on osteoblasts are dependent on estrogen receptor alpha signaling and cytoskeletal remodeling.[Pubmed:30093109]
Biochem Biophys Res Commun. 2018 Sep 18;503(4):2678-2684.
Clinical and experimental studies demonstrate the potential of low-magnitude high-frequency vibration (LMHFV) to enhance bone formation in the intact skeleton and during fracture healing. Moreover, it was shown that the effects of vibration therapy during fracture healing are highly dependent on the estrogen status of the vibrated individual and that estrogen receptor (ER) alpha signaling plays a major role in mechanotransduction of LMHFV. Because it is known that LMHFV can directly act on osteogenic cells, we hypothesize that the differential effects of LMHFV in the presence and absence of estrogen are mediated by ERalpha signaling in osteoblasts. To prove this hypothesis, we subjected preosteoblastic MC3T3-E1 cells and primary osteoblasts to LMHFV in vitro. We found increased Cox2 gene expression, cell metabolic activity and cell proliferation after LMHFV in the absence of estrogen, whereas the effects were contrary in the presence of estrogen. Blocking of ERalpha signaling by Esr1-siRNA knockdown or adding the selective ERalpha antagonist MPP dihydrochloride abolished the effects of LMHFV on osteoblast proliferation and Cox2 expression. Furthermore, primary osteoblasts isolated from ERalpha-knockout mice did not show a response towards LMHFV in the presence of estrogen. Additionally, blocking of actin cytoskeletal remodeling by adding the p160ROCK inhibitor Y-27632 abolished the effects of LMHFV. In contrast, expression of primary cilium was not necessary for mechanotransduction of LMHFV. These results suggest that direct effects of LMHFV on osteoblasts are dependent on ERalpha signaling and cytoskeletal remodeling.
Rapid estrogen receptor-alpha signaling mediated by ERK activation regulates vascular tone in male and ovary-intact female mice.[Pubmed:28887333]
Am J Physiol Heart Circ Physiol. 2018 Feb 1;314(2):H330-H342.
Estrogen has been shown to affect vascular reactivity. Here, we assessed the estrogen receptor-alpha (ERalpha) dependency of estrogenic effects on vasorelaxation via a rapid nongenomic pathway in both male and ovary-intact female mice. We compared the effect of a primary estrogen, 17beta-estradiol (E2) or 4,4',4''-(4-propyl-[1H]pyrazole-1,3,5-triyl)tris-phenol (PPT; selective ERalpha agonist). We found that E2 and PPT induced greater aortic relaxation in female mice than in male mice, indicating ERalpha mediation, which was further validated by using ERalpha antagonism. Treatment with 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (MPP dihydrochloride; ERalpha antagonist) attenuated PPT-mediated vessel relaxation in both sexes. ERalpha-mediated vessel relaxation was further validated by the absence of significant PPT-mediated relaxation in aortas isolated from ERalpha knockout mice. Treatment with a specific ERK inhibitor, PD-98059, reduced E2-induced vessel relaxation in both sexes but to a lesser extent in female mice. Furthermore, PD-98059 prevented PPT-induced vessel relaxation in both sexes. Both E2 and PPT treatment activated ERK as early as 5-10 min, which was attenuated by PD-98059 in aortic tissue, cultured primary vascular smooth muscle cells (VSMCs), and endothelial cells (ECs). Aortic rings denuded of endothelium showed no differences in vessel relaxation after E2 or PPT treatment, implicating a role of ECs in the observed sex differences. Here, our results are unique to show estrogen-stimulated rapid ERalpha signaling mediated by ERK activation in aortic tissue, as well as VSMCs and ECs in vitro, in regulating vascular function by using side-by-side comparisons in male and ovary-intact female mice in response to E2 or PPT. NEW & NOTEWORTHY Here, we assessed the estrogen receptor-alpha dependency of estrogenic effects in vasorelaxation of both male and ovary-intact female mice by performing side-by-side comparisons. Also, we describe the connection between estrogen-stimulated rapid estrogen receptor-alpha signaling and downstream ERK activation in regulating vascular function in male and ovary-intact female mice.
Estrogen receptors and estetrol-dependent neuroprotective actions: a pilot study.[Pubmed:27799463]
J Endocrinol. 2017 Jan;232(1):85-95.
Estetrol (E4) has strong antioxidative, neurogenic and angiogenic effects in neural system resulting in the attenuation of neonatal hypoxic-ischemic encephalopathy. We aimed to define the role of estrogen receptors in E4-dependent actions in neuronal cell cultures and prove the promyelinating effect of E4. In vitro the antioxidative and cell survival/proliferating effects of E4 on H2O2-induced oxidative stress in primary hippocampal cell cultures were studied using different combinations of specific inhibitors for ERalpha (MPP dihydrochloride), ERbeta (PHTTP), GPR30 (G15) and palmytoilation (2-BR). LDH activity and cell survival assays were performed. In vivo the promyelinating role of different concentrations of E4 (1 mg/kg/day, 5 mg/kg/day, 10 mg/kg/day, 50 mg/kg/day) was investigated using the hypoxic-ischemic brain damage model in the 7-day-old immature rats before/after the induction of hypoxic-ischemic insult. Myelin basic protein (MBP) immunostaining was performed on brain coronal sections. Our results show that LDH activity is significantly upregulated in cell cultures where the E4's effect was completely blocked by concomitant treatment either with ERalpha and ERbeta inhibitors (MPP and PHTPP, respectively), or ERalpha and ERbeta inhibitors combined with 2-BR. Cell survival is significantly downregulated in cell cultures where the effect of E4 was blocked by ERbeta inhibitor (PHTTP) alone. The blockage of GRP30 receptor did affect neither LDH activity nor cell survival. MBP immunostaining is significantly upregulated in E4-pretreated groups at a concentration of 5 mg/kg/day and 50 mg/kg/day E4, whereas the MBP-positive area OD ratio is significantly increased in all the E4-treated groups. E4's antioxidative actions mostly depend on ERalpha and ERbeta, whereas neurogenesis and possibly promyelinating activities might be realized through ERbeta.
Activities of estrogen receptor alpha- and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression.[Pubmed:12943986]
Mol Cell Endocrinol. 2003 Aug 29;206(1-2):13-22.
Estrogens exert their regulatory transcriptional effects, which can be stimulatory or repressive, at diverse gene sites via two estrogen receptors, ERalpha and ERbeta. Since these two ERs have different tissue distributions, ligands that have the capacity to selectively activate or inhibit these two ERs would be useful in elucidating the biology of these two receptors and might assist in the development of estrogen pharmaceuticals with improved tissue selectivity. We have developed several ligands that showed ERalpha or ERbeta selectivity at promoter-gene sites containing consensus estrogen response elements (EREs): ERalpha-selective agonist (propyl-pyrazole-triol (PPT)), ERalpha-selective antagonist (methyl-piperidino-pyrazole (MPP)), ERbeta-potency selective agonist (diarylpropionitrile (DPN)) and ERbeta-selective antagonist/ERalpha-agonist (R,R-tetrahydrochrysene (R,R-THC)). In this study, we have examined the activity of these compounds at a range of gene sites where ER stimulates gene expression through non-consensus EREs (complement C3), or multiple half-EREs (NHE-RF/EBP50), or by tethering to DNA via other proteins (TGF beta3 and progesterone receptor A/AP-1), and at gene sites where ER represses gene transcription (interleukin-6). At all of these genes, PPT showed full stimulation through ERalpha while displaying no agonism through ERbeta. MPP antagonized estradiol actions on gene transactivation and transrepression through ERalpha, with little or no effect on transcription mediated through ERbeta. DPN displayed subtype-selective agonism, being ca. 30-fold more potent through ERbeta. R,R-THC was a complete antagonist through ERbeta and displayed agonism through ERalpha, the level of which was promoter dependent. Because these ligands maintain their agonist or antagonist character and ER subtype-selectivity at gene sites of diverse nature, where estradiol is either stimulatory or inhibitory, these compounds should prove useful in elucidating the biological functions of ERalpha and ERbeta.


