InfractinCAS# 91147-07-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 91147-07-8 | SDF | Download SDF |
| PubChem ID | 5319542 | Appearance | Cryst. |
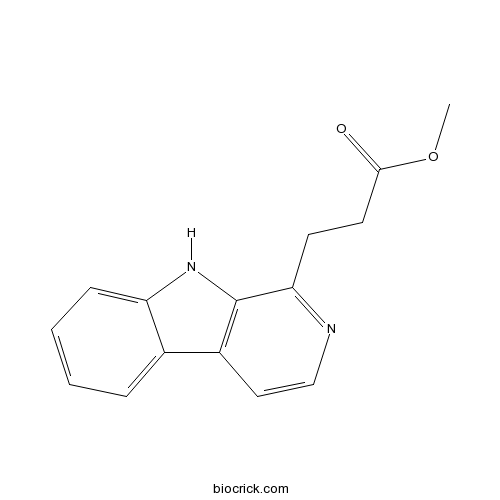
| Formula | C15H14N2O2 | M.Wt | 254.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl 3-(9H-pyrido[3,4-b]indol-1-yl)propanoate | ||
| SMILES | COC(=O)CCC1=NC=CC2=C1NC3=CC=CC=C23 | ||
| Standard InChIKey | IYWBIIGDWQBZJQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H14N2O2/c1-19-14(18)7-6-13-15-11(8-9-16-13)10-4-2-3-5-12(10)17-15/h2-5,8-9,17H,6-7H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Liebigs Annalen der Chemie, 1993, 1993(2).Synthesis of Manzamine C, Infractine and 6‐Hydroxyinfractine.[Reference: WebLink]
|

Infractin Dilution Calculator

Infractin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9324 mL | 19.6618 mL | 39.3236 mL | 78.6473 mL | 98.3091 mL |
| 5 mM | 0.7865 mL | 3.9324 mL | 7.8647 mL | 15.7295 mL | 19.6618 mL |
| 10 mM | 0.3932 mL | 1.9662 mL | 3.9324 mL | 7.8647 mL | 9.8309 mL |
| 50 mM | 0.0786 mL | 0.3932 mL | 0.7865 mL | 1.5729 mL | 1.9662 mL |
| 100 mM | 0.0393 mL | 0.1966 mL | 0.3932 mL | 0.7865 mL | 0.9831 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- SLx-2119
Catalog No.:BCC1954
CAS No.:911417-87-3
- EC 144
Catalog No.:BCC5600
CAS No.:911397-80-3
- MPP dihydrochloride
Catalog No.:BCC7225
CAS No.:911295-24-4
- LY2603618
Catalog No.:BCC3923
CAS No.:911222-45-2
- Cefoselis hydrochloride
Catalog No.:BCC4093
CAS No.:911212-25-4
- Adapalene sodium salt
Catalog No.:BCC4285
CAS No.:911110-93-5
- SB 706504
Catalog No.:BCC5615
CAS No.:911110-38-8
- Isotussilagine
Catalog No.:BCN1985
CAS No.:91108-32-6
- Furowanin A
Catalog No.:BCN4790
CAS No.:911004-72-3
- 3,6,19,23-Tetrahydroxy-12-ursen-28-oic acid
Catalog No.:BCN1310
CAS No.:91095-51-1
- 8-Epidiosbulbin E acetate
Catalog No.:BCN7812
CAS No.:91095-48-6
- Danshenol C
Catalog No.:BCN6681
CAS No.:910856-25-6
- Terbinafine
Catalog No.:BCC3865
CAS No.:91161-71-6
- 17-AAG Hydrochloride
Catalog No.:BCC1297
CAS No.:911710-03-7
- Chrysothol
Catalog No.:BCN4454
CAS No.:911714-91-5
- Lucyoside B
Catalog No.:BCN7811
CAS No.:91174-19-5
- Ophiopogonin C
Catalog No.:BCN5379
CAS No.:911819-08-4
- H-Met-OtBu.HCl
Catalog No.:BCC2996
CAS No.:91183-71-0
- Noscapine HCl
Catalog No.:BCC3819
CAS No.:912-60-7
- Spantide I
Catalog No.:BCC5808
CAS No.:91224-37-2
- Karavilagenin A
Catalog No.:BCN4455
CAS No.:912329-03-4
- TCB-2
Catalog No.:BCC7421
CAS No.:912342-28-0
- ABT-888 (Veliparib)
Catalog No.:BCC1267
CAS No.:912444-00-9
- Veliparib dihydrochloride
Catalog No.:BCC2076
CAS No.:912445-05-7
[A new indole alkaloid from the stems of Brucea mollis].[Pubmed:24761613]
Yao Xue Xue Bao. 2014 Feb;49(2):225-9.
Eight compounds were isolated from the stems of Brucea mollis by various chromatographic techniques such as column chromatography on silica gel and Sephadex LH-20, and preparative HPLC, and their structures were elucidated as bruceolline O (1), 1-(1-beta-glucopyranosyl)-1H-indole-3-carbaldehyde (2), canthin-6-one (3), 11-hydroxycanthin-6-one (4), 9-methoxycanthin-6-one (5), 4-methoxycanthin-6-one (6), Infractin (7), and beta-carboline-1-propionic acid (8). The cytotoxic activities of compounds 1-8 against HCT-8 and A549 human cell lines were determined, but none of them exhibited significant activity (IC 50 > 10 micromol x L(-1)). Among them, compound 1 is a new indole alkaloid, and compounds 2 and 5-7 were isolated from this plant for the first time.


