LY341495Metabotropic glutamate receptor antagonist CAS# 201943-63-7 |
- MTEP hydrochloride
Catalog No.:BCC1780
CAS No.:1186195-60-7
- mGlu2 agonist
Catalog No.:BCC1745
CAS No.:1311385-32-6
- CTEP (RO4956371)
Catalog No.:BCC4599
CAS No.:871362-31-1
- Dipraglurant
Catalog No.:BCC1531
CAS No.:872363-17-2
- MPEP
Catalog No.:BCC4594
CAS No.:96206-92-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 201943-63-7 | SDF | Download SDF |
| PubChem ID | 9819927 | Appearance | Powder |
| Formula | C20H19NO5 | M.Wt | 353.38 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 6 mg/mL (16.98 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
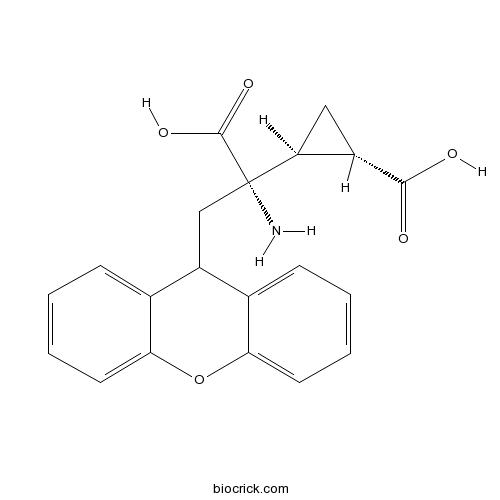
| Chemical Name | (1S,2S)-2-[(1S)-1-amino-1-carboxy-2-(9H-xanthen-9-yl)ethyl]cyclopropane-1-carboxylic acid | ||
| SMILES | C1C(C1C(CC2C3=CC=CC=C3OC4=CC=CC=C24)(C(=O)O)N)C(=O)O | ||
| Standard InChIKey | VLZBRVJVCCNPRJ-KPHUOKFYSA-N | ||
| Standard InChI | InChI=1S/C20H19NO5/c21-20(19(24)25,15-9-13(15)18(22)23)10-14-11-5-1-3-7-16(11)26-17-8-4-2-6-12(14)17/h1-8,13-15H,9-10,21H2,(H,22,23)(H,24,25)/t13-,15-,20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Highly potent and selective group II metabotropic glutamate receptor antagonist (Ki/IC50 values are 2.3, 1.3, 173, 990, 6800, 8200 and 22000 nM for human mGlu2, mGlu3, mGlu8 , mGlu7a, mGlu1a, mGlu5a and mGlu4a receptors respectively). Readily brain penetrant and active in vivo. Disodium salt also available. Also available as part of the Group II mGlu Receptor, Group III mGlu Receptor and Mixed mGlu Receptor |

LY341495 Dilution Calculator

LY341495 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8298 mL | 14.1491 mL | 28.2981 mL | 56.5963 mL | 70.7454 mL |
| 5 mM | 0.566 mL | 2.8298 mL | 5.6596 mL | 11.3193 mL | 14.1491 mL |
| 10 mM | 0.283 mL | 1.4149 mL | 2.8298 mL | 5.6596 mL | 7.0745 mL |
| 50 mM | 0.0566 mL | 0.283 mL | 0.566 mL | 1.1319 mL | 1.4149 mL |
| 100 mM | 0.0283 mL | 0.1415 mL | 0.283 mL | 0.566 mL | 0.7075 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LY341495 is a highly potent and selective antagonist of group II metabotropic glutamate receptors with IC50 values of 21 nM and 14 nM for mGlu2 and mGlu3 receptors, respectively [1].
Glutamate is the major excitatory neurotransmitter that regulates neuronal plasticity and maintains fast synaptic transmission. The aberrant glutamate pathway has been found in a serious of neurological?problems, such as cognitive disorders, epilepsy and AD. As a selective inhibitor of group II metabotropic glutamate receptors (including mGlu2 and mGlu3), LY341495 acts as a competitive antagonist to regulate the transmission of glutamate [1 and 2].
In the functional assays, LY341495 at concentrations of 0.1 μM and 30 nM completely reversed the ACPD-induced inhibition of cAMP formation which was stimulated by forskolin in mGlu2 expressing cells and mGlu3 expressing cells, respectively. In RGT cells expressing group III mGlu receptors (mGlu4, 6, 7 and 8), LY341495 showed significant reversion of L-AP4-inhibited cAMP formation stimulated by forskolin. For mGlu4a, 7a and 8, the IC50 value of LY341495 were 22, 0.99 and 0.173 μM, respectively, which were 5 to 1000-fold higher than that for mGlu2 and mGlu3. For the group I mGlu receptors (mGlu1a and 5a), the IC50 values were 6.8 and 8.2 μM, respectively. Besides that, LY341495 was found to reversibly eliminate the long-term synaptic depression induced by DHPG in rats’ hippocampal slices [1 and 2].
In animal experiments, LY341495 was found to have effects on the post-training recognition memory. It was reported that the administration of LY341495 at different doses showed different efficacies on rats’ recognition memory. Rats treated with LY341495 at doses of 0.3, 1 and 3 mg/kg displayed a significant lower level of discrimination while LY341495 at doses of 0.05 and 0.1 mg/kg exerted a reversion of the recognition memory extinction and showed no impairment of the recognition memory [3].
References:
[1] Kingston A E, Ornstein P L, Wright R A, et al. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology, 1998, 37(1): 1-12.
[2] Fitzjohn S M, Bortolotto Z A, Palmer M J, et al. The potent mGlu receptor antagonist LY341495 identifies roles for both cloned and novel mGlu receptors in hippocampal synaptic plasticity. Neuropharmacology, 1998, 37(12): 1445-1458.
[3] Pitsikas N, Kaffe E, Markou A. The metabotropic glutamate 2/3 receptor antagonist LY341495 differentially affects recognition memory in rats. Behavioural brain research, 2012, 230(2): 374-379.
- Epicatechin pentaacetate
Catalog No.:BCN4884
CAS No.:20194-41-6
- PTAC oxalate
Catalog No.:BCC6217
CAS No.:201939-40-4
- Ombuoside
Catalog No.:BCN3711
CAS No.:20188-85-6
- Magnolioside
Catalog No.:BCN2832
CAS No.:20186-29-2
- Pisatin
Catalog No.:BCN3912
CAS No.:20186-22-5
- Tenuifolin
Catalog No.:BCN5005
CAS No.:20183-47-5
- Z-Leu-OH
Catalog No.:BCC2766
CAS No.:2018-66-8
- Ac-Phe-OH
Catalog No.:BCC3005
CAS No.:2018-61-3
- Isodiospyrin
Catalog No.:BCN4883
CAS No.:20175-84-2
- (S)-3,4-DCPG
Catalog No.:BCC7012
CAS No.:201730-11-2
- (R)-3,4-DCPG
Catalog No.:BCC7046
CAS No.:201730-10-1
- Triptocalline A
Catalog No.:BCN6783
CAS No.:201534-10-3
- Kaempferol 7-O-rhamnoside
Catalog No.:BCN6489
CAS No.:20196-89-8
- Tenofovir Disoproxil Fumarate
Catalog No.:BCC1108
CAS No.:202138-50-9
- Bilastine
Catalog No.:BCC5263
CAS No.:202189-78-4
- Spiperone hydrochloride
Catalog No.:BCC6882
CAS No.:2022-29-9
- Flucytosine
Catalog No.:BCC3780
CAS No.:2022-85-7
- Spiraeoside
Catalog No.:BCC8251
CAS No.:20229-56-5
- Dregeoside Aa1
Catalog No.:BCN4678
CAS No.:20230-41-5
- Dasycarpol
Catalog No.:BCN7134
CAS No.:202343-57-5
- PNU-159682
Catalog No.:BCC5463
CAS No.:202350-68-3
- Scillascillol
Catalog No.:BCN7008
CAS No.:2023822-39-9
- Scillascillone
Catalog No.:BCN6992
CAS No.:2023822-40-2
- Scillascilloside B-1
Catalog No.:BCN6998
CAS No.:2023822-41-3
Antidepressant-like effects of scopolamine in mice are enhanced by the group II mGlu receptor antagonist LY341495.[Pubmed:27569995]
Neuropharmacology. 2016 Dec;111:169-179.
Clinical studies have shown that the muscarinic receptor antagonist scopolamine induces a potent and rapid antidepressant effect relative to conventional antidepressants. However, potential undesirable effects, including memory impairment, partially limit the use of scopolamine in psychiatry. In the present study, we propose to overcome these limitations and enhance the therapeutic effects of scopolamine via administration in combination with the group II metabotropic glutamate (mGlu) receptor antagonist, LY341495. Joint administration of sub-effective doses of scopolamine (0.03 or 0.1 mg/kg, i.p.) with a sub-effective dose of LY341495 (0.1 mg/kg, i.p.) induced a profound antidepressant effect in the tail suspension test (TST) and in the forced swim test (FST) in mice. This drug combination did not impair memory, as measured using the Morris water maze (MWM), and did not influence the locomotor activity of mice. Furthermore, we found that an AMPA receptor antagonist, NBQX (10 mg/kg), completely reversed the antidepressant-like activity of a mixture of scopolamine and LY341495 in the TST. However, this effect was not influenced by para-chlorophenylalanine (PCPA) pre-treatment, indicating a lack of involvement of serotonergic system activation in the antidepressant-like effects of jointly given scopolamine and LY341495. Therefore, the combined administration of low doses of the antimuscarinic drug scopolamine and the group II mGlu receptor antagonist LY341495 might be a new, effective and safe strategy in the therapy of depression.
Group II mGlu receptor antagonist LY341495 enhances the antidepressant-like effects of ketamine in the forced swim test in rats.[Pubmed:27286960]
Psychopharmacology (Berl). 2016 Aug;233(15-16):2901-14.
RATIONALE: Numerous preclinical and clinical studies have reported the rapid and sustained antidepressant effects of the NMDA receptor antagonist ketamine. Because ketamine induces several undesirable and dangerous effects, a variety of strategies have been suggested to avoid such effects. OBJECTIVES: Here, we propose to enhance the sub-effective doses of ketamine by co-administration with the group II metabotropic glutamate (mGlu) receptor antagonist LY341495. This compound potentially acts as an antidepressant via a mechanism similar to that of ketamine. METHODS: To investigate the rapid and sustained antidepressant-like effects of these drugs, we administered ketamine and LY341495 individually or in combination, 40 min and 24 h before the forced swim test (FST). RESULTS: We found that sub-effective doses of ketamine and LY341495, given jointly, induce significant antidepressant-like effects, at both 40 min and 24 h after administration. The results obtained using Western blot technique indicate that mammalian target of rapamycin (mTOR) pathway activation may be involved in the mechanism of this action. The effects of drugs, used at identical ranges of times and doses, on spontaneous locomotor activity in rats were excluded. Furthermore, the results obtained from the rota-rod test and the ketamine-induced hyperlocomotion test suggest a lack of potentially adverse effects from the combined administration of ketamine and LY341495 at doses previously used in the FST. CONCLUSION: Altogether, these data suggest that the joint administration of ketamine and LY341495 might be a noteworthy alternative to the use of solely ketamine in the therapy of depression.
The Rapidly Acting Antidepressant Ketamine and the mGlu2/3 Receptor Antagonist LY341495 Rapidly Engage Dopaminergic Mood Circuits.[Pubmed:27189960]
J Pharmacol Exp Ther. 2016 Jul;358(1):71-82.
Ketamine is a rapidly acting antidepressant in patients with treatment-resistant depression (TRD). Although the mechanisms underlying these effects are not fully established, inquiry to date has focused on the triggering of synaptogenesis transduction pathways via glutamatergic mechanisms. Preclinical data suggest that blockade of metabotropic glutamate (mGlu2/3) receptors shares many overlapping features and mechanisms with ketamine and may also provide rapid efficacy for TRD patients. Central dopamine circuitry is recognized as an end target for mood regulation and hedonic valuation and yet has been largely neglected in mechanistic studies of antidepressant-relevant effects of ketamine. Herein, we evaluated the changes in dopaminergic neurotransmission after acute administration of ketamine and the mGlu2/3 receptor antagonist LY341495 [(2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid ] in preclinical models using electrophysiologic, neurochemical, and behavioral endpoints. When given acutely, both ketamine and LY341495, but not the selective serotonin reuptake inhibitor (SSRI) citalopram, increased the number of spontaneously active dopamine neurons in the ventral tegmental area (VTA), increased extracellular levels of dopamine in the nucleus accumbens and prefrontal cortex, and enhanced the locomotor stimulatory effects of the dopamine D2/3 receptor agonist quinpirole. Further, both ketamine and LY341495 reduced immobility time in the tail-suspension assay in CD1 mice, which are relatively resistant to SSRI antidepressants. Both the VTA neuronal activation and the antidepressant phenotype induced by ketamine and LY341495 were attenuated by the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo- (9CI)-benzo[f]quinoxaline-7-sulfonamide, indicating AMPA-dependent effects. These findings provide another overlapping mechanism of action of ketamine and mGlu2/3 receptor antagonism that differentiates them from conventional antidepressants and thus support the potential rapidly acting antidepressant actions of mGlu2/3 receptor antagonism in patients.
Lower [3H]LY341495 binding to mGlu2/3 receptors in the anterior cingulate of subjects with major depressive disorder but not bipolar disorder or schizophrenia.[Pubmed:26521087]
J Affect Disord. 2016 Jan 15;190:241-248.
INTRODUCTION: The glutamatergic system has recently been implicated in the pathogenesis and treatment of major depressive disorders(MDD) and mGlu2/3 receptors play an important role in regulating glutamatergic tone. We therefore measured cortical levels of mGlu2/3 to determine if they were changed in MDD. METHODS: Binding parameters for [(3)H]LY341495 (mGlu2/3 antagonist) were determined to allow optimized in situ binding with autoradiography to be completed using a number of CNS regions. Subsequently, density of [(3)H]LY341495 binding was measured in BA24(anterior cingulate cortex), BA17(visual cortex) and BA46(dorsolateral prefrontal cortex) from subjects with MDD, Bipolar Disorder(BPD), Schizophrenia(SCZ), and controls, as well as rats treated with imipramine (20mg/kg), fluoxetine (10mg/kg), or vehicle. RESULTS: mGlu2/3 are widely expressed throughout the brain with high levels observed in cortex. [(3)H]LY341495 binding was significantly lower in BA24 from subjects with MDD (mean +/- SEM=141.3 +/- 14.65 fmol/ETE) relative to controls (184.9 +/- 7.76 fmol/ETE; Cohen's d=1.005, p<0.05). There were no other differences with diagnoses, and chronic antidepressant treatment in rats had minimal effect on binding. LIMITATIONS: Using this approach we are unable to determine whether the change represents fluctuations in mGlu2, mGlu3, or both. Moreover, using postmortem tissue we are unable to dissociate the irrevocable confound of suicidality upon binding levels. CONCLUSION: We have demonstrated lower [(3)H]LY341495 binding levels in MDD in BA24-a brain region implicated in depression. Moreover we show that the lower levels are unlikely to be the result of antidepressant treatment. These data suggest that levels of either mGlu2 and/or mGlu3 are affected in the aetiology of MDD.
[3H]-LY341495 as a novel antagonist radioligand for group II metabotropic glutamate (mGlu) receptors: characterization of binding to membranes of mGlu receptor subtype expressing cells.[Pubmed:10530814]
Neuropharmacology. 1999 Oct;38(10):1519-29.
Metabotropic glutamate (mGlu) receptors are a family of eight known subtypes termed mGlu1-8. Currently, few ligands are available to study the pharmacology of mGlu receptor subtypes. In functional assays, we previously described LY341495 as a highly potent and selective mGlu2 and mGlu3 receptor antagonist. In this study, radiolabeled [3H]-LY341495 was used to investigate the characteristics of receptor binding to membranes from cells expressing human mGlu receptor subtypes. Using membranes from cells expressing human mGlu2 and mGlu3 receptors, [3H]-LY341495 (1 nM) specific binding was > 90% of total binding. At an approximate K(D) concentration for [3H]-LY341495 binding to human mGlu2 and mGlu3 receptors (1 nM), no appreciable specific binding of [3H-]LY341495 was found in membranes of cells expressing human mGlu1a, mGlu5a, mGlu4a, mGlu6, or mGlu7a receptors. However, modest (approximately 20% of mGlu2/3) specific [3H]-LY341495 (1 nM) binding was observed in human mGlu8 expressing cells. [3H]-LY341495 bound to membranes expressing human mGlu2 and mGlu3 receptors in a reversible and saturable manner with relatively high affinities (Bmax 20.5 +/- 5.4 and 32.0 +/- 7.0 pmol/mg protein; and K(D) = 1.67 +/- 0.20 and 0.75 +/- 0.43 nM, respectively). The pharmacology of [3H]-LY341495 binding in mGlu2 and mGlu3 expressing cells was consistent with that previously described for LY341495 in functional assays. [3H]-LY341495 binding provides a useful way to further investigate regulation of receptor expression and pharmacological properties of mGlu2 and mGlu3 receptor subtypes in recombinant systems.
LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors.[Pubmed:9680254]
Neuropharmacology. 1998;37(1):1-12.
The in vitro pharmacology of a structurally novel compound, LY341495, was investigated at human recombinant metabotropic glutamate (mGlu) receptor subtypes expressed in non-neuronal (RGT, rat glutamate transporter) cells. LY341495 was a nanomolar potent antagonist of 1S,3R-1-aminocyclopentane-1,3-dicarboxylic acid (ACPD)-induced inhibition of forskolin-stimulated cAMP formation at mGlu2 and mGlu3 receptors (respective IC50S of 0.021 and 0.014 microM). At group I mGlu receptor expressing cells, LY341495 was micromolar potent in antagonizing quisqualate-induced phosphoinositide (PI) hydrolysis, with IC50 values of 7.8 and 8.2 microM for mGlu1a and mGlu5a receptors, respectively. Among the human group III mGlu receptors, the most potent inhibition of L-2-amino-4-phosphonobutyric acid (L-AP4) responses was seen for LY341495 at mGlu8, with an IC50 of 0.17 microM. LY341495 was less potent at mGlu7 (IC50 = 0.99 microM) and least potent at mGlu4 (IC50 = 22 microM). Binding studies in rat brain membranes also demonstrated nanomolar potent group II mGlu receptor affinity for LY341495, with no appreciable displacement of ionotropic glutamate receptor ligand binding. Thus, LY341495 has a unique range of selectivity across the mGlu receptor subtypes with a potency order of mGlu3 > or = mGlu2 > mGlu8 > mGlu7 >> mGlu1a = mGlu5a > mGlu4. In particular, LY341495 is the most potent antagonist yet reported at mGlu2, 3 and 8 receptors. Thus, it represents a novel pharmacological agent for elucidating the function of mGlu receptors in experimental systems.
2-substituted (2SR)-2-amino-2-((1SR,2SR)-2-carboxycycloprop-1-yl)glycines as potent and selective antagonists of group II metabotropic glutamate receptors. 2. Effects of aromatic substitution, pharmacological characterization, and bioavailability.[Pubmed:9464367]
J Med Chem. 1998 Jan 29;41(3):358-78.
In this paper we describe the synthesis of a series of alpha-substituted analogues of the potent and selective group II metabotropic glutamate receptor (mGluR) agonist (1S,1'S,2'S)-carboxycyclopropylglycine (2, L-CCG 1). Incorporation of a substitutent on the amino acid carbon converted the agonist 2 into an antagonist. All of the compounds were prepared and tested as a series of four isomers, i.e., two racemic diastereomers. On the basis of the improvement in affinity realized for the alpha-phenylethyl analogue 3, in this paper we explored the effects of substitution on the aromatic ring as a strategy to increase the affinity to these compounds for group II mGluRs. Affinity for group II mGluRs was measured using [3H]glutamic acid (Glu) binding in rat forebrain membranes. Antagonist activity was confirmed for these compounds by measuring their ability to antagonize (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid-induced inhibition of forskolin stimulated cyclic-AMP in RGT cells transfected with human mGluR2 and mGluR3. Meta substitution on the aromatic ring of 3 with a variety of substituents, both electron donating (e.g., methyl, hydroxy, amino, methoxy, phenyl, phenoxy) and electron withdrawing (e.g., fluorine, chlorine, bromine, carboxy, trifluoromethyl) gave from 1.5- to 4.5-fold increases in affinity. Substitution with p-fluorine, as in 97 (IC50 = 0.022 +/- 0.002), was the exception. Here, a greater increase in affinity was realized than for either the ortho- or meta-substituted analogues; 97 was the most potent compound resulting from monosubstitution of the aromatic. At best, only modest increases in affinity were realized for certain compounds bearing either two chlorines or two fluorines, and two methoxy groups gave no improvement in affinity (all examined in a variety of substitution patterns). Three amino acids, 4, 5, and 104, were resolved into their four constituent isomers, and affinity and functional activity for group II mGluRs was found to reside solely in the S,S,S-isomers of each, consistent with 1. With an IC50 = 2.9 +/- 0.6 nM, the resolved xanthylmethyl compound 168 was the most potent compound from this SAR. Amino acid 168 demonstrated high plasma levels following intraperitoneal (i.p.) administration and readily penetrated into the brain. This compound, however, had only limited (approximately 5%) oral bioavailability. Systemic administration of 168 protected mice from limbic seizures produced by the mGluR agonist 3,5-dihydroxyphenylglycine, with an ED50 = 31 mg/kg (i.p., 60 min preinjection). Thus, 168 represents a valuable tool to study the role of group II mGluRs in disease.


