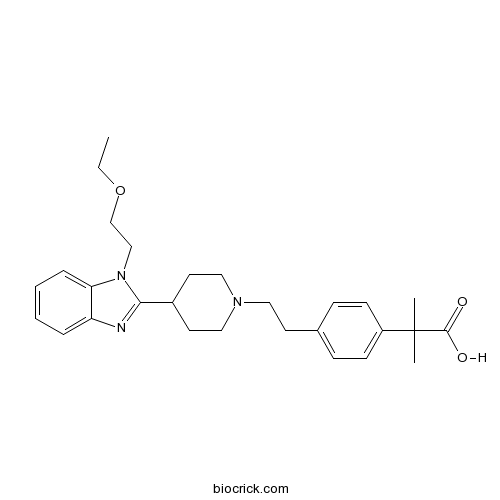
BilastineCAS# 202189-78-4 |
- CHIR-99021 (CT99021)
Catalog No.:BCC1275
CAS No.:252917-06-9
- SB 415286
Catalog No.:BCC3651
CAS No.:264218-23-7
- SB 216763
Catalog No.:BCC3650
CAS No.:280744-09-4
- AR-A014418
Catalog No.:BCC1366
CAS No.:487021-52-3
- LY2090314
Catalog No.:BCC1717
CAS No.:603288-22-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 202189-78-4 | SDF | Download SDF |
| PubChem ID | 185460 | Appearance | Powder |
| Formula | C28H37N3O3 | M.Wt | 463.61 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 49.3 mg/mL (106.34 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-[4-[2-[4-[1-(2-ethoxyethyl)benzimidazol-2-yl]piperidin-1-yl]ethyl]phenyl]-2-methylpropanoic acid | ||
| SMILES | CCOCCN1C2=CC=CC=C2N=C1C3CCN(CC3)CCC4=CC=C(C=C4)C(C)(C)C(=O)O | ||
| Standard InChIKey | ACCMWZWAEFYUGZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C28H37N3O3/c1-4-34-20-19-31-25-8-6-5-7-24(25)29-26(31)22-14-17-30(18-15-22)16-13-21-9-11-23(12-10-21)28(2,3)27(32)33/h5-12,22H,4,13-20H2,1-3H3,(H,32,33) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Bilastine Dilution Calculator

Bilastine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.157 mL | 10.7849 mL | 21.5699 mL | 43.1397 mL | 53.9246 mL |
| 5 mM | 0.4314 mL | 2.157 mL | 4.314 mL | 8.6279 mL | 10.7849 mL |
| 10 mM | 0.2157 mL | 1.0785 mL | 2.157 mL | 4.314 mL | 5.3925 mL |
| 50 mM | 0.0431 mL | 0.2157 mL | 0.4314 mL | 0.8628 mL | 1.0785 mL |
| 100 mM | 0.0216 mL | 0.1078 mL | 0.2157 mL | 0.4314 mL | 0.5392 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tenofovir Disoproxil Fumarate
Catalog No.:BCC1108
CAS No.:202138-50-9
- Kaempferol 7-O-rhamnoside
Catalog No.:BCN6489
CAS No.:20196-89-8
- LY341495
Catalog No.:BCC1724
CAS No.:201943-63-7
- Epicatechin pentaacetate
Catalog No.:BCN4884
CAS No.:20194-41-6
- PTAC oxalate
Catalog No.:BCC6217
CAS No.:201939-40-4
- Ombuoside
Catalog No.:BCN3711
CAS No.:20188-85-6
- Magnolioside
Catalog No.:BCN2832
CAS No.:20186-29-2
- Pisatin
Catalog No.:BCN3912
CAS No.:20186-22-5
- Tenuifolin
Catalog No.:BCN5005
CAS No.:20183-47-5
- Z-Leu-OH
Catalog No.:BCC2766
CAS No.:2018-66-8
- Ac-Phe-OH
Catalog No.:BCC3005
CAS No.:2018-61-3
- Isodiospyrin
Catalog No.:BCN4883
CAS No.:20175-84-2
- Spiperone hydrochloride
Catalog No.:BCC6882
CAS No.:2022-29-9
- Flucytosine
Catalog No.:BCC3780
CAS No.:2022-85-7
- Spiraeoside
Catalog No.:BCC8251
CAS No.:20229-56-5
- Dregeoside Aa1
Catalog No.:BCN4678
CAS No.:20230-41-5
- Dasycarpol
Catalog No.:BCN7134
CAS No.:202343-57-5
- PNU-159682
Catalog No.:BCC5463
CAS No.:202350-68-3
- Scillascillol
Catalog No.:BCN7008
CAS No.:2023822-39-9
- Scillascillone
Catalog No.:BCN6992
CAS No.:2023822-40-2
- Scillascilloside B-1
Catalog No.:BCN6998
CAS No.:2023822-41-3
- 2,2-Bis(4-chloroformyloxyphenyl)propane
Catalog No.:BCC8491
CAS No.:2024-88-6
- Etoricoxib
Catalog No.:BCC1565
CAS No.:202409-33-4
- Hydroxygenkwanin
Catalog No.:BCN4885
CAS No.:20243-59-8
Bilastine in allergic rhinoconjunctivitis and urticaria: a practical approach to treatment decisions based on queries received by the medical information department.[Pubmed:28210286]
Drugs Context. 2017 Feb 3;6:212500.
BACKGROUND: Bilastine is a safe and effective commonly prescribed non-sedating H1-antihistamine approved for symptomatic treatment in patients with allergic disorders such as rhinoconjunctivitis and urticaria. It was evaluated in many patients throughout the clinical development required for its approval, but clinical trials generally exclude many patients who will benefit in everyday clinical practice (especially those with coexisting diseases and/or being treated with concomitant drugs). Following its introduction into clinical practice, the Medical Information Specialists at Faes Farma have received many practical queries regarding the optimal use of Bilastine in different circumstances. DATA SOURCES AND METHODS: Queries received by the Medical Information Department and the responses provided to senders of these queries. RESULTS: The most frequent questions received by the Medical Information Department included the potential for drug-drug interactions with Bilastine and commonly used agents such as anticoagulants (including the novel oral anticoagulants), antiretrovirals, antituberculosis regimens, corticosteroids, digoxin, oral contraceptives, and proton pump inhibitors. Most of these medicines are not usually allowed in clinical trials, and so advice needs to be based upon the pharmacological profiles of the drugs involved and expert opinion. The pharmacokinetic profile of Bilastine appears favourable since it undergoes negligible metabolism and is almost exclusively eliminated via renal excretion, and it neither induces nor inhibits the activity of several isoenzymes from the CYP 450 system. Consequently, Bilastine does not interact with cytochrome metabolic pathways. Other queries involved specific patient groups such as subjects with renal impairment, women who are breastfeeding or who are trying to become pregnant, and patients with other concomitant diseases. Interestingly, several questions related to topics that are well covered in the Summary of Product Characteristics (SmPC), which suggests that this resource is not being well used. CONCLUSIONS: Overall, this analysis highlights gaps in our knowledge regarding the optimal use of Bilastine. Expert opinion based upon an understanding of the science can help in the decision-making, but more research is needed to provide evidence-based answers in certain circumstances.
One-year safety and efficacy study of bilastine treatment in Japanese patients with chronic spontaneous urticaria or pruritus associated with skin diseases.[Pubmed:27862227]
J Dermatol. 2017 Apr;44(4):375-385.
A number of second-generation non-sedating antihistamines are used in clinical practices over the world. However, long-term safety and efficacy have not been proved high level evidence based medicine. We have performed an open-label, multicenter, phase III study to evaluate the long-term safety and efficacy of Bilastine, a novel non-sedating H1 -antihistamine for patients with chronic spontaneous urticaria (CSU) or pruritus associated with skin diseases (trial registration no. JapicCTI-142528). Patients aged 18-74 years were treated with Bilastine 20 mg once daily for up to 52 weeks. Safety and tolerability were assessed on the basis of adverse events (AE), Bilastine-related AE, laboratory tests and vital signs. Efficacy was assessed based on rash score, itch score, overall improvement and quality of life. One hundred and ninety-eight patients enrolled, 122 of whom (61.6%) completed the 52-week treatment period. AE were reported in 64.5% and Bilastine-related AE in 2.5% of patients throughout the 52-week treatment period. All AE were mild to moderate in severity. AE associated with the nervous system occurred in 10 patients (5.1%) including seven patients (3.6%) with headache. Somnolence reported in two of these patients (1.0%) was related to Bilastine. All efficacy variables improved during treatment with Bilastine. In conclusion, long-term treatment with Bilastine 20 mg once daily for 52 weeks is safe and well tolerated in Japanese patients with CSU or pruritus associated with skin diseases. Bilastine improved disease symptoms of both conditions early in treatment, and the efficacy was maintained throughout the treatment.


