CHIR-99021 (CT99021)GSK-3 inhibitor, Cell-permeable, ATP-competitive CAS# 252917-06-9 |
Quality Control & MSDS
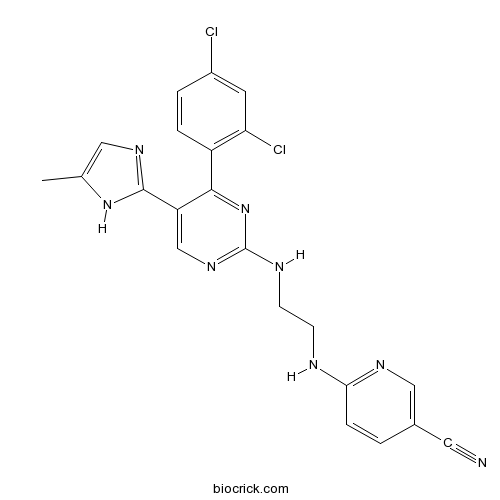
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 252917-06-9 | SDF | Download SDF |
| PubChem ID | 9956119 | Appearance | Powder |
| Formula | C22H18Cl2N8 | M.Wt | 465.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CT99021 | ||
| Solubility | DMSO : 16.67 mg/mL (35.82 mM; Need ultrasonic) | ||
| Chemical Name | 6-[2-[[4-(2,4-dichlorophenyl)-5-(5-methyl-1H-imidazol-2-yl)pyrimidin-2-yl]amino]ethylamino]pyridine-3-carbonitrile | ||
| SMILES | CC1=CN=C(N1)C2=CN=C(N=C2C3=C(C=C(C=C3)Cl)Cl)NCCNC4=NC=C(C=C4)C#N | ||
| Standard InChIKey | AQGNHMOJWBZFQQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H18Cl2N8/c1-13-10-29-21(31-13)17-12-30-22(32-20(17)16-4-3-15(23)8-18(16)24)27-7-6-26-19-5-2-14(9-25)11-28-19/h2-5,8,10-12H,6-7H2,1H3,(H,26,28)(H,29,31)(H,27,30,32) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and highly selective inhibitor of glycogen synthase kinase 3 (GSK-3) (IC50 values are 6.7 and 10 nM for GSK-3β and GSK-3α respectively). Exhibits >500-fold selectivity for GSK-3 over closely related kinases; also displays >800-fold selectivity against 45 additional enzymes and receptors. In combination with ItemId=248590'>tranylcypromine 3852), enables reprogramming of mouse embryonic fibroblasts, transduced by Oct4 and Klf4 only, into iPSCs. Enhances mouse and human ESC self-renewal when used in combination with PD 0325901. |

CHIR-99021 (CT99021) Dilution Calculator

CHIR-99021 (CT99021) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.149 mL | 10.7448 mL | 21.4897 mL | 42.9793 mL | 53.7242 mL |
| 5 mM | 0.4298 mL | 2.149 mL | 4.2979 mL | 8.5959 mL | 10.7448 mL |
| 10 mM | 0.2149 mL | 1.0745 mL | 2.149 mL | 4.2979 mL | 5.3724 mL |
| 50 mM | 0.043 mL | 0.2149 mL | 0.4298 mL | 0.8596 mL | 1.0745 mL |
| 100 mM | 0.0215 mL | 0.1074 mL | 0.2149 mL | 0.4298 mL | 0.5372 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CHIR-99021 is a highly specific glycogen synthase kinase-3 (GSK-3) inhibitor which can inhibit both isoforms with IC50 of 10 nM (GSK-3α) and 6.7 nM (GSK-3β).
CHIR-99021 was proved to promote self-renewal and maintain pluripotency of both B6 and BALB/c ES cells via stabilizing the downstream effectors like c-Myc and β -catenin[1]. In J1 mESC cells, CHIR-99021 played an important role in maintaining the colony morphology as well as the self-renewal when combined with leukemia inhibitory factor (LIF). CHIR-99021 has shown to regulate multiple signaling pathways which involve Wnt/β-catenin, TGF-β, Nodal and MAPK, and the expression of epigenetic regulatory genes like Dnmt3l[2].
CHIR-99021 has been demonstrated to promote DN3 thymocytes proliferation and differentiation in the absence of pre-TCR signaling, Notch1 signaling or CXCL12[3]. However, study has also found that higher concentration (10 ?M but not 1 ?M or 3 ?M) of CHIR99021 might selectively inhibit differentiation by activating IL-7 signaling pathway[3].
References:
1. Ye S1, Tan L, Yang R, Fang B, Qu S, Schulze EN, Song H, Ying Q, Li P. Pleiotropy of glycogen synthase kinase-3 inhibition by CHIR99021 promotes self-renewal of embryonic stem cells from refractory mouse strains. PLoS One. 2012;7(4):e35892.
2. Wu Y1, Ai Z, Yao K, Cao L, Du J, Shi X, Guo Z, Zhang Y. CHIR99021 promotes self-renewal of mouse embryonic stem cells by modulation of protein-encoding gene and long intergenic non-coding RNA expression. Exp Cell Res. 2013
3. PLoS One. 2013;8(3):e58501. doi: 10.1371/journal.pone.0058501. Epub 2013 Mar 20. Schroeder JH1, Bell LS, Janas ML, Turner M. Pharmacological inhibition of glycogen synthase kinase 3 regulates T cell development in vitro. PLoS One. 2013;8(3):e58501.
- TSU-68 (SU6668,Orantinib)
Catalog No.:BCC2508
CAS No.:252916-29-3
- RWJ 56110
Catalog No.:BCC7433
CAS No.:252889-88-6
- 20S,24R-Epoxydammar-12,25-diol-3-one
Catalog No.:BCN5118
CAS No.:25279-15-6
- Aristolene
Catalog No.:BCN8417
CAS No.:6831-16-9
- Apelin-36 (human)
Catalog No.:BCC5910
CAS No.:252642-12-9
- Ethyl orsellinate
Catalog No.:BCN4662
CAS No.:2524-37-0
- Lucidal
Catalog No.:BCN3206
CAS No.:252351-96-5
- Lucidadiol
Catalog No.:BCN7142
CAS No.:252351-95-4
- Isotaxiresinol 9,9'-acetonide
Catalog No.:BCN4663
CAS No.:252333-72-5
- 9,9'-O-Isopropyllidene-isolariciresinol
Catalog No.:BCN1474
CAS No.:252333-71-4
- 1-Cinnamoylpyrrole
Catalog No.:BCN4006
CAS No.:252248-89-8
- Tertiapin-Q
Catalog No.:BCC5740
CAS No.:252198-49-5
- CHIR-98014
Catalog No.:BCC3751
CAS No.:252935-94-7
- MRE 3008F20
Catalog No.:BCC6106
CAS No.:252979-43-4
- Luteosporin
Catalog No.:BCN5390
CAS No.:2530-39-4
- Eribulin
Catalog No.:BCC5174
CAS No.:253128-41-5
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Tetrabromobisphenol A diallyl ether
Catalog No.:BCC9174
CAS No.:25327-89-3
- Isocalamendiol
Catalog No.:BCN5119
CAS No.:25330-21-6
- BMS 195614
Catalog No.:BCC7740
CAS No.:253310-42-8
- Trazodone HCl
Catalog No.:BCC5032
CAS No.:25332-39-2
- Viniferin
Catalog No.:BCN3757
CAS No.:253435-07-3
- (+)-Corlumidine
Catalog No.:BCN2662
CAS No.:25344-54-1
- Wiskostatin
Catalog No.:BCC7934
CAS No.:253449-04-6
Inhibiting glycogen synthase kinase-3 and transforming growth factor-beta signaling to promote epithelial transition of human adipose mesenchymal stem cells.[Pubmed:28698144]
Biochem Biophys Res Commun. 2017 Sep 2;490(4):1381-1388.
BACKGROUND: This study was aimed to investigate the epithelial differentiation of human adipose-derived mesenchymal stem cells (ADSCs) by inhibiting glycogen synthase kinase-3 (GSK3) and transforming growth factor beta (TGFbeta) signaling. METHODS AND RESULTS: STEMPRO human ADSCs at passage 2 were treated with CHIR99021 (GSK3 inhibitor), E-616452 (TGFbeta1 receptor kinase inhibitor), A-83-01 (TGFbeta type 1 receptor inhibitor), valproic acid (histone deacetylase inhibitor), tranylcypromine (monoamine oxidase inhibitor) and all-trans retinoic acid for 72 h. The mesenchymal-epithelial transition was shown by down-regulation of mesenchymal genes (Slug, Zinc Finger E-box Binding Homeobox 1 ZEB1, integrin alpha5 ITGA5 and vimentin VIM) and up-regulation of epithelial genes (E-cadherin, Epithelial Cell Adhesion Molecule EpCAM, Zonula Occludens-1 ZO-1, occludin, deltaN p63 deltaNp63, Transcription Factor 4 TCF4 and Twist Family bHLH Transcription Factor TWIST), compared to untreated ADSCs. Cell morphology and stress fiber pattern were examined and the treated cells became less migratory in scratch wound closure assay. The formation of cell junction complexes was observed under transmission electron microscopy. Global gene expression using GeneChip((R)) Human Genome U133 Array (Affymetrix) showed that the treatment up-regulated 540 genes (containing genes for cell cycle, cytoskeleton reorganization, chemotaxis, epithelium development and regulation of cell migration) and down-regulated 483 genes. CONCLUSION: Human ADSCs were transited to epithelial lineage by inhibiting GSK3 and TGFbeta signaling. It can be an adult stem cell source for epithelial cell-based therapy.
Hyperglycemia impedes definitive endoderm differentiation of human embryonic stem cells by modulating histone methylation patterns.[Pubmed:28283910]
Cell Tissue Res. 2017 Jun;368(3):563-578.
Exposure to maternal diabetes during fetal growth is a risk factor for the development of type II diabetes (T2D) in later life. Discovery of the mechanisms involved in this association should provide valuable background for therapeutic treatments. Early embryogenesis involves epigenetic changes including histone modifications. The bivalent histone methylation marks H3K4me3 and H3K27me3 are important for regulating key developmental genes during early fetal pancreas specification. We hypothesized that maternal hyperglycemia disrupted early pancreas development through changes in histone bivalency. A human embryonic stem cell line (VAL3) was used as the cell model for studying the effects of hyperglycemia upon differentiation into definitive endoderm (DE), an early stage of the pancreatic lineage. Hyperglycemic conditions significantly down-regulated the expression levels of DE markers SOX17, FOXA2, CXCR4 and EOMES during differentiation. This was associated with retention of the repressive histone methylation mark H3K27me3 on their promoters under hyperglycemic conditions. The disruption of histone methylation patterns was observed as early as the mesendoderm stage, with Wnt/beta-catenin signaling being suppressed during hyperglycemia. Treatment with Wnt/beta-catenin signaling activator CHIR-99021 restored the expression levels and chromatin methylation status of DE markers, even in a hyperglycemic environment. The disruption of DE development was also found in mouse embryos at day 7.5 post coitum from diabetic mothers. Furthermore, disruption of DE differentiation in VAL3 cells led to subsequent impairment in pancreatic progenitor formation. Thus, early exposure to hyperglycemic conditions hinders DE development with a possible relationship to the later impairment of pancreas specification.
The Role of Sequential BMP Signaling in Directing Human Embryonic Stem Cells to Bipotential Gonadal Cells.[Pubmed:28938435]
J Clin Endocrinol Metab. 2017 Nov 1;102(11):4303-4314.
Context: Human gonads arise as a pair of epithelial ridges on the surface of intermediate mesoderm (IM)-derived mesonephros. Toxic environmental factors and mutations in various genes are known to disturb normal gonadal development, but because of a lack of suitable in vitro models, detailed studies characterizing the molecular basis of the observed defects have not been performed. Objective: To establish an in vitro method for studying differentiation of bipotential gonadal progenitors by using human embryonic stem cells (hESCs) and to investigate the role of bone morphogenetic protein (BMP) in gonadal differentiation. Design: We tested 17 protocols using activin A, CHIR-99021, and varying durations of BMP-7 and the BMP inhibitor dorsomorphin. Activation of activin A, WNT, and BMP pathways was optimized to induce differentiation. Setting: Academic research laboratory. Main Outcomes Measures: Cell differentiation, gene expression, and flow cytometry. Results: The two most efficient protocols consistently upregulated IM markers LHX1, PAX2, and OSR1 at days 2 to 4 and bipotential gonadal markers EMX2, GATA4, WT1, and LHX9 at day 8 of culture. The outcome depended on the combination of the duration, concentration, and type of BMP activation and the length of WNT signaling. Adjusting any of the parameters substantially affected the requirements for other parameters. Conclusions: We have established a reproducible protocol for directed differentiation of hESCs into bipotential gonadal cells. The protocol can be used to model early gonadal development in humans and allows further differentiation to mature gonadal somatic cells.
Baicalein Inhibits the Proliferation of Cervical Cancer Cells Through the GSK3beta-Dependent Pathway.[Pubmed:28835320]
Oncol Res. 2018 May 7;26(4):645-653.
Baicalein, a flavonoid derived from the root of Scutellaria baicalensis, has been reported to possess multiple pharmacological activities, such as anticancer and anti-inflammatory properties. This study investigated the effect of baicalein in cervical cancer cells. Cell growth curve and MTT assay were performed and revealed that baicalein inhibited the proliferation of SiHa and HeLa cells in a dose-dependent manner. We further found that baicalein arrested the cell cycle of SiHa and HeLa cells at the G0/G1 phase by suppressing the expression of cyclin D1 through the downregulation of phosphorylated protein kinase B (p-AKT) and phosphorylated glycogen synthase kinase 3beta (p-GSK3beta) according to FACS assays and Western blotting. Moreover, when CHIR-99021, a GSK3beta inhibitor, was added to baicalein-treated SiHa cells, the expression of cyclin D1 was recovered, and cell proliferation was promoted. In conclusion, these data indicated that baicalein suspended the cell cycle at the G0/G1 phase via the downregulation of cyclin D1 through the AKT-GSK3beta signaling pathway and further inhibited the proliferation of SiHa and HeLa cervical cancer cells.
Brief exposure to small molecules allows induction of mouse embryonic fibroblasts into neural crest-like precursors.[Pubmed:28129669]
FEBS Lett. 2017 Feb;591(4):590-602.
In this study, we propose a novel method for inducing neuronal cells by briefly exposing them to small-molecule cocktails in a step-by-step manner. Global gene expression analysis with immunohistochemical staining and calcium flux assays reveal the generation of neurons from mouse embryonic fibroblasts. In addition, time-lapse imaging of neural precursor-specific enhancer expression and global gene expression analyses show that the neurons are generated by passing through a neural crest-like precursor stage. Consistent with these results, the neural crest-like cells are able to differentiate into neural crest lineage cells, such as sympathetic neurons, adipocytes, osteocytes, and smooth muscle cells. Therefore, these results indicate that brief exposure to chemical compounds could expand and induce a substantial multipotent cell population without viral transduction.
Pleiotropy of glycogen synthase kinase-3 inhibition by CHIR99021 promotes self-renewal of embryonic stem cells from refractory mouse strains.[Pubmed:22540008]
PLoS One. 2012;7(4):e35892.
BACKGROUND: Inhibition of glycogen synthase kinase-3 (GSK-3) improves the efficiency of embryonic stem (ES) cell derivation from various strains of mice and rats, as well as dramatically promotes ES cell self-renewal potential. beta-catenin has been reported to be involved in the maintenance of self-renewal of ES cells through TCF dependent and independent pathway. But the intrinsic difference between ES cell lines from different species and strains has not been characterized. Here, we dissect the mechanism of GSK-3 inhibition by CHIR99021 in mouse ES cells from refractory mouse strains. METHODOLOGY/PRINCIPAL FINDINGS: We found that CHIR99021, a GSK-3 specific inhibitor, promotes self-renewal of ES cells from recalcitrant C57BL/6 (B6) and BALB/c mouse strains through stabilization of beta-catenin and c-Myc protein levels. Stabilized beta-catenin promoted ES self-renewal through two mechanisms. First, beta-catenin translocated into the nucleus to maintain stem cell pluripotency in a lymphoid-enhancing factor/T-cell factor-independent manner. Second, beta-catenin binds plasma membrane-localized E-cadherin, which ensures a compact, spherical morphology, a hallmark of ES cells. Further, elevated c-Myc protein levels did not contribute significantly to CH-mediated ES cell self-renewal. Instead, the role of c-Myc is dependent on its transformation activity and can be replaced by N-Myc but not L-Myc. beta-catenin and c-Myc have similar effects on ES cells derived from both B6 and BALB/c mice. CONCLUSIONS/SIGNIFICANCE: Our data demonstrated that GSK-3 inhibition by CH promotes self-renewal of mouse ES cells with non-permissive genetic backgrounds by regulation of multiple signaling pathways. These findings would be useful to improve the availability of normally non-permissive mouse strains as research tools.
AKT kinase activity is required for lithium to modulate mood-related behaviors in mice.[Pubmed:21389981]
Neuropsychopharmacology. 2011 Jun;36(7):1397-411.
Bipolar disorder (BP) is a debilitating psychiatric disorder, affecting approximately 2% of the worldwide population, for which the etiological basis, pathogenesis, and neurocircuitry remain poorly understood. Individuals with BP suffer from recurrent episodes of mania and depression, which are commonly treated with the mood stabilizer lithium. However, nearly half of BP patients do not respond adequately to lithium therapy and the clinically relevant mechanisms of lithium for mood stabilization remain elusive. Here, we modeled lithium responsiveness using cellular assays of glycogen synthase kinase 3 (GSK-3) signaling and mood-related behavioral assays in inbred strains of mice that differ in their response to lithium. We found that activating AKT through phosphosrylation of a key regulatory site (Thr308) was associated with lithium response-activation of signaling pathways downstream of GSK-3 in cells and attenuation of mood-related behaviors in mice-and this response was attenuated by selective and direct inhibition of AKT kinase activity. Conversely, the expression of constitutively active AKT1 in both the cellular and behavioral assays conferred lithium sensitivity. In contrast, selective and direct GSK-3 inhibition by the ATP-competitive inhibitor CHIR99021 bypassed the requirement for AKT activation and modulated behavior in both lithium-responsive and non-responsive mouse strains. These results distinguish the mechanism of action of lithium from direct GSK-3 inhibition both in vivo and in vitro, and highlight the therapeutic potential for selective GSK-3 inhibitors in BP treatment.
Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2.[Pubmed:19839055]
Stem Cells. 2009 Dec;27(12):2992-3000.
Induced pluripotent stem cell technology has attracted enormous interest for potential application in regenerative medicine. Here, we report that a specific glycogen synthase kinase 3 (GSK-3) inhibitor, CHIR99021, can induce the reprogramming of mouse embryonic fibroblasts transduced by only two factors, Oct4 and Klf4. When combined with Parnate (also named tranylcypromine), an inhibitor of lysine-specific demethylase 1, CHIR99021 can cause the reprogramming of human primary keratinocyte transduced with the two factors, Oct4 and Klf4. To our knowledge, this is the first time that human iPS cells have been generated from somatic cells without exogenous Sox2 expression. Our studies suggest that the GSK-3 inhibitor might have a general application to replace transcription factors in both mouse and human reprogramming.
Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo.[Pubmed:12606497]
Diabetes. 2003 Mar;52(3):588-95.
Insulin resistance plays a central role in the development of type 2 diabetes, but the precise defects in insulin action remain to be elucidated. Glycogen synthase kinase 3 (GSK-3) can negatively regulate several aspects of insulin signaling, and elevated levels of GSK-3 have been reported in skeletal muscle from diabetic rodents and humans. A limited amount of information is available regarding the utility of highly selective inhibitors of GSK-3 for the modification of insulin action under conditions of insulin resistance. In the present investigation, we describe novel substituted aminopyrimidine derivatives that inhibit human GSK-3 potently (K(i) < 10 nmol/l) with at least 500-fold selectivity against 20 other protein kinases. These low molecular weight compounds activated glycogen synthase at approximately 100 nmol/l in cultured CHO cells transfected with the insulin receptor and in primary hepatocytes isolated from Sprague-Dawley rats, and at 500 nmol/l in isolated type 1 skeletal muscle of both lean Zucker and ZDF rats. It is interesting that these GSK-3 inhibitors enhanced insulin-stimulated glucose transport in type 1 skeletal muscle from the insulin-resistant ZDF rats but not from insulin-sensitive lean Zucker rats. Single oral or subcutaneous doses of the inhibitors (30-48 mg/kg) rapidly lowered blood glucose levels and improved glucose disposal after oral or intravenous glucose challenges in ZDF rats and db/db mice, without causing hypoglycemia or markedly elevating insulin. Collectively, our results suggest that these selective GSK-3 inhibitors may be useful as acute-acting therapeutics for the treatment of the insulin resistance of type 2 diabetes.


