Apelin-36 (human)Endogenous APJ receptor agonist CAS# 252642-12-9 |
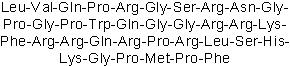
- LDK378
Catalog No.:BCC3691
CAS No.:1032900-25-6
- LDK378 dihydrochloride
Catalog No.:BCC1694
CAS No.:1380575-43-8
- TAE684 (NVP-TAE684)
Catalog No.:BCC3660
CAS No.:761439-42-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 252642-12-9 | SDF | Download SDF |
| PubChem ID | 131954567 | Appearance | Powder |
| Formula | C184H297N69O43S | M.Wt | 4195.87 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
| Sequence | LVQPRGSRNGPGPWQGGRRKFRRQRPRLSH | ||
| Chemical Name | 2-[[1-[2-[[1-[2-[[6-amino-2-[[2-[[2-[[2-[[2-[[1-[2-[[5-amino-2-[[2-[[2-[[2-[[6-amino-2-[[2-[[2-[[2-[[2-[[5-amino-2-[[2-[[1-[2-[[1-[2-[[4-amino-2-[[2-[[2-[[2-[[2-[[1-[5-amino-2-[[2-[(2-amino-4-methylpentanoyl)amino]-3-methylbutanoyl]amino]-5-oxopentanoyl]pyrrolidine-2-carbonyl]amino]-5-carbamimidamidopentanoyl]amino]acetyl]amino]-3-hydroxypropanoyl]amino]-5-carbamimidamidopentanoyl]amino]-4-oxobutanoyl]amino]acetyl]pyrrolidine-2-carbonyl]amino]acetyl]pyrrolidine-2-carbonyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-5-oxopentanoyl]amino]acetyl]amino]acetyl]amino]-5-carbamimidamidopentanoyl]amino]-5-carbamimidamidopentanoyl]amino]hexanoyl]amino]-3-phenylpropanoyl]amino]-5-carbamimidamidopentanoyl]amino]-5-carbamimidamidopentanoyl]amino]-5-oxopentanoyl]amino]-5-carbamimidamidopentanoyl]pyrrolidine-2-carbonyl]amino]-5-carbamimidamidopentanoyl]amino]-4-methylpentanoyl]amino]-3-hydroxypropanoyl]amino]-3-(1H-imidazol-4-yl)propanoyl]amino]hexanoyl]amino]acetyl]pyrrolidine-2-carbonyl]amino]-4-methylsulfanylbutanoyl]pyrrolidine-2-carbonyl]amino]-3-phenylpropanoic acid | ||
| SMILES | CC(C)CC(C(=O)NC(C(C)C)C(=O)NC(CCC(=O)N)C(=O)N1CCCC1C(=O)NC(CCCNC(=N)N)C(=O)NCC(=O)NC(CO)C(=O)NC(CCCNC(=N)N)C(=O)NC(CC(=O)N)C(=O)NCC(=O)N2CCCC2C(=O)NCC(=O)N3CCCC3C(=O)NC(CC4=CNC5=CC=CC=C54)C(=O)NC(CCC(=O)N)C(=O)NCC(=O)NCC(=O)NC(CCCNC(=N)N)C(=O)NC(CCCNC(=N)N)C(=O)NC(CCCCN)C(=O)NC(CC6=CC=CC=C6)C(=O)NC(CCCNC(=N)N)C(=O)NC(CCCNC(=N)N)C(=O)NC(CCC(=O)N)C(=O)NC(CCCNC(=N)N)C(=O)N7CCCC7C(=O)NC(CCCNC(=N)N)C(=O)NC(CC(C)C)C(=O)NC(CO)C(=O)NC(CC8=CNC=N8)C(=O)NC(CCCCN)C(=O)NCC(=O)N9CCCC9C(=O)NC(CCSC)C(=O)N1CCCC1C(=O)NC(CC1=CC=CC=C1)C(=O)O)N | ||
| Standard InChIKey | IRSHPNNQNFBJMK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C184H297N69O43S/c1-98(2)80-106(187)147(266)247-146(100(5)6)172(291)239-120(58-61-138(190)258)174(293)252-77-32-53-133(252)169(288)235-109(42-20-65-209-177(192)193)148(267)220-91-142(262)226-128(95-254)164(283)232-115(47-25-70-214-182(202)203)157(276)243-126(86-139(191)259)151(270)222-93-143(263)248-73-28-50-130(248)166(285)223-94-145(265)250-75-30-52-132(250)168(287)244-124(84-103-87-217-107-39-15-14-38-105(103)107)162(281)233-117(56-59-136(188)256)150(269)219-89-140(260)218-90-141(261)225-110(43-21-66-210-178(194)195)152(271)229-112(44-22-67-211-179(196)197)153(272)228-111(41-17-19-64-186)156(275)241-123(82-101-34-10-8-11-35-101)161(280)231-114(46-24-69-213-181(200)201)154(273)230-113(45-23-68-212-180(198)199)155(274)234-118(57-60-137(189)257)159(278)237-119(49-27-72-216-184(206)207)173(292)251-76-31-54-134(251)170(289)236-116(48-26-71-215-183(204)205)158(277)240-122(81-99(3)4)160(279)246-129(96-255)165(284)242-125(85-104-88-208-97-224-104)163(282)227-108(40-16-18-63-185)149(268)221-92-144(264)249-74-29-51-131(249)167(286)238-121(62-79-297-7)175(294)253-78-33-55-135(253)171(290)245-127(176(295)296)83-102-36-12-9-13-37-102/h8-15,34-39,87-88,97-100,106,108-135,146,217,254-255H,16-33,40-86,89-96,185-187H2,1-7H3,(H2,188,256)(H2,189,257)(H2,190,258)(H2,191,259)(H,208,224)(H,218,260)(H,219,269)(H,220,267)(H,221,268)(H,222,270)(H,223,285)(H,225,261)(H,226,262)(H,227,282)(H,228,272)(H,229,271)(H,230,273)(H,231,280)(H,232,283)(H,233,281)(H,234,274)(H,235,288)(H,236,289)(H,237,278)(H,238,286)(H,239,291)(H,240,277)(H,241,275)(H,242,284)(H,243,276)(H,244,287)(H,245,290)(H,246,279)(H,247,266)(H,295,296)(H4,192,193,209)(H4,194,195,210)(H4,196,197,211)(H4,198,199,212)(H4,200,201,213)(H4,202,203,214)(H4,204,205,215)(H4,206,207,216) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous APJ receptor agonist (EC50 = 20 nM) that is secreted by adipocytes. Binds with high affinity to human APJ receptors expressed in HEK 293 cells (pIC50= 8.61). Involved in regulation of cardiovascular function, fluid homeostasis and feeding. Blocks entry of some HIV-1 and HIV-2 strains into NP-2/CD4 cells expressing APJ. |

Apelin-36 (human) Dilution Calculator

Apelin-36 (human) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ethyl orsellinate
Catalog No.:BCN4662
CAS No.:2524-37-0
- Lucidal
Catalog No.:BCN3206
CAS No.:252351-96-5
- Lucidadiol
Catalog No.:BCN7142
CAS No.:252351-95-4
- Isotaxiresinol 9,9'-acetonide
Catalog No.:BCN4663
CAS No.:252333-72-5
- 9,9'-O-Isopropyllidene-isolariciresinol
Catalog No.:BCN1474
CAS No.:252333-71-4
- 1-Cinnamoylpyrrole
Catalog No.:BCN4006
CAS No.:252248-89-8
- Tertiapin-Q
Catalog No.:BCC5740
CAS No.:252198-49-5
- Fmoc-Lys(Me2)-OH
Catalog No.:BCC2567
CAS No.:252049-10-8
- Fmoc-D-Threoninol
Catalog No.:BCC2702
CAS No.:252049-02-8
- AZD7545
Catalog No.:BCC4294
CAS No.:252017-04-2
- A 205804
Catalog No.:BCC3944
CAS No.:251992-66-2
- SLV 320
Catalog No.:BCC7656
CAS No.:251945-92-3
- Aristolene
Catalog No.:BCN8417
CAS No.:6831-16-9
- 20S,24R-Epoxydammar-12,25-diol-3-one
Catalog No.:BCN5118
CAS No.:25279-15-6
- RWJ 56110
Catalog No.:BCC7433
CAS No.:252889-88-6
- TSU-68 (SU6668,Orantinib)
Catalog No.:BCC2508
CAS No.:252916-29-3
- CHIR-99021 (CT99021)
Catalog No.:BCC1275
CAS No.:252917-06-9
- CHIR-98014
Catalog No.:BCC3751
CAS No.:252935-94-7
- MRE 3008F20
Catalog No.:BCC6106
CAS No.:252979-43-4
- Luteosporin
Catalog No.:BCN5390
CAS No.:2530-39-4
- Eribulin
Catalog No.:BCC5174
CAS No.:253128-41-5
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Tetrabromobisphenol A diallyl ether
Catalog No.:BCC9174
CAS No.:25327-89-3
- Isocalamendiol
Catalog No.:BCN5119
CAS No.:25330-21-6
The bioactive peptides salusins and apelin-36 are produced in human arterial and venous tissues and the changes of their levels during cardiopulmonary bypass.[Pubmed:22884920]
Peptides. 2012 Oct;37(2):233-9.
This study aimed to examine the effects of CPB on salusin-alpha, salusin-beta and apelin-36 bioactive peptides in people who are planned to undergo coronary artery bypass graft (CABG) operation due to coronary artery disease and to explore whether these peptides are produced in human aortic, saphenous and arterial tissues. The study included age and BMI matched 15 patients who underwent CABG operation by CPB. In order to determine salusin-alpha, salusin-beta and apelin-36 levels, venous blood samples were collected before induction of anesthesia (T1), before CPB (T2), 5 min before the removal of cross-clamp (T3), 5 min after the removal of cross-clamp (T4), upon arrival in the intensive care (T5), at postoperative 24th hour (T6) and 72nd hour (T7). Salusin and apelin expressions of the tissues were shown by immunohistochemical method. Peptide amounts of sera and tissues were measured using ELISA. Salusins production by vessels occurs in fibroblast cells of the media in the aorta and smooth muscle cells of the media in the LIMA and saphena. Apelin is produced by endothelial cells of the intima and fibroblast cells of the media in the aorta and by smooth muscle cells of the media in the LIMA and saphena. Changes in the levels of salusin-beta and apelin-36 were significant during CPB. Salusin-alpha, salusin-beta and apelin-36 are locally synthesized in the arteries and veins. Salusins and apelin-36 might be important markers in the CPB, and also that salusin-beta was more specific in comparison to salusin-alpha.
Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin.[Pubmed:12603839]
J Neurochem. 2003 Mar;84(5):1162-72.
Apelin peptides have recently been identified to be the endogenous ligands for the G protein-coupled receptor APJ. However, little is known about the physiological roles of this ligand-receptor pairing. In the present study we investigated the pharmacology of several apelin analogues at the human recombinant APJ receptor using radioligand binding and functional assays. This has led to the identification of key residues in the apelin peptide required for functional potency and binding affinity through structure-activity studies. In particular, we have identified that replacement of leucine in position 5, or arginine in position 2 and 4 of the C-terminal apelin peptide, apelin-13, resulted in significant changes in pharmacology. We also investigated the detailed localization of pre-proapelin and APJ receptor mRNA in a wide range of human, rat and mouse tissues using quantitative RT-PCR, and carried out a detailed immunohistochemical study of the distribution of the APJ receptor in rat brain and spinal cord. Interestingly, the APJ receptor was not only co-localized in white matter with GFAP in the spinal cord, but was also clearly localized on neurones in the brain, suggesting that this receptor and its peptide may be involved in a wide range of biological process yet to be determined.
Apelin peptides block the entry of human immunodeficiency virus (HIV).[Pubmed:10802050]
FEBS Lett. 2000 May 4;473(1):15-8.
The orphan G protein-coupled receptor APJ has been shown to be a coreceptor for human and simian immunodeficiency virus (HIV and SIV) strains. We have determined that some HIV and SIV strains use APJ as a coreceptor to infect the brain-derived NP-2/CD4 cells. Because apelin is an endogenous ligand for the APJ receptor, we examined the inhibitory effects of apelin peptides on HIV infection, and found that the apelin peptides inhibit the entry of some HIV-1 and HIV-2 into the NP-2/CD4 cells expressing APJ. The inhibitory efficiency has been found to be in the order of apelin-36>apelin-17>apelin-13>apelin-12.


