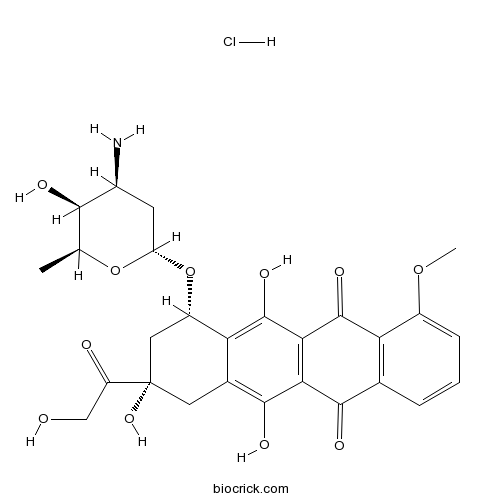
Doxorubicin (Adriamycin) HClAntitumour antibiotic,inhibits TOPO II. CAS# 25316-40-9 |
- WR 1065
Catalog No.:BCC2417
CAS No.:14653-77-1
- Arctiin
Catalog No.:BCN1090
CAS No.:20362-31-6
- Daunorubicin
Catalog No.:BCC4115
CAS No.:20830-81-3
- Daunorubicin HCl
Catalog No.:BCC5083
CAS No.:23541-50-6
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Epirubicin HCl
Catalog No.:BCC1192
CAS No.:56390-09-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 25316-40-9 | SDF | Download SDF |
| PubChem ID | 443939 | Appearance | Powder |
| Formula | C27H30ClNO11 | M.Wt | 579.98 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Adriamycin, NSC 123127,Doxorubicin hydrochloride | ||
| Solubility | DMSO : 50 mg/mL (86.21 mM; Need ultrasonic) H2O : 33.33 mg/mL (57.47 mM; Need ultrasonic) | ||
| Chemical Name | (7S,9S)-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride | ||
| SMILES | CC1C(C(CC(O1)OC2CC(CC3=C(C4=C(C(=C23)O)C(=O)C5=C(C4=O)C=CC=C5OC)O)(C(=O)CO)O)N)O.Cl | ||
| Standard InChIKey | MWWSFMDVAYGXBV-RUELKSSGSA-N | ||
| Standard InChI | InChI=1S/C27H29NO11.ClH/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34;/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3;1H/t10-,13-,15-,17-,22+,27-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antitumor antibiotic agent that inhibits DNA topoisomerase II. DNA intercalator that inhibits nucleic acid synthesis and induces apoptosis. Reduces intracellular tau levels. |

Doxorubicin (Adriamycin) HCl Dilution Calculator

Doxorubicin (Adriamycin) HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7242 mL | 8.621 mL | 17.242 mL | 34.4839 mL | 43.1049 mL |
| 5 mM | 0.3448 mL | 1.7242 mL | 3.4484 mL | 6.8968 mL | 8.621 mL |
| 10 mM | 0.1724 mL | 0.8621 mL | 1.7242 mL | 3.4484 mL | 4.3105 mL |
| 50 mM | 0.0345 mL | 0.1724 mL | 0.3448 mL | 0.6897 mL | 0.8621 mL |
| 100 mM | 0.0172 mL | 0.0862 mL | 0.1724 mL | 0.3448 mL | 0.431 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Doxorubicin is a semi-synthesized anticancer agent derived from bacterial culture. [1] It is an anthracycline antibiotic. It is been widely used in blood cancers, solid tumors and sarcomas.
Doxorubicin intercalates into DNA double strand and inhibits the progression of DNA topoisomerase II, stopping replication process. [2] Doxorubicin also induces histone eviction from open chromatin, causing DNA damage and epigenetic deregulation. [3]
Doxorubicin is administrated intravenously. Approximately 75% of doxorubicin and its metabolites bind to plasma protein. Doxorubicin does not cross blood brain barrier. 50% of the drug is eliminated unchanged from the body mainly though bile excretion. The remaining undergoes one-electron reduction, two-electron reduction, and deglycosidation. The major metabolite is a potent membrane ion pump inhibitor, which is associated with cardiomyopathy. [4]
References:
[1]Brayfield, A, ed. (2013). Doxorubicin. Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved 15 April 2014.
[2]Pommier Y., et al. (2010). DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chemistry & Biology 17 (5): 421–433.
[3]Pang, B., et al. (2013). Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin. Nature Communications 4 (5): 1908
[4]Boucek RJ., et al. (1987). The major metabolite of doxorubicin is a potent inhibitor of membrane-associated ion pumps. A correlative study of cardiac muscle with isolated membrane fractions. J of Biol Chem 262: 15851-15856.
- Eribulin
Catalog No.:BCC5174
CAS No.:253128-41-5
- Luteosporin
Catalog No.:BCN5390
CAS No.:2530-39-4
- MRE 3008F20
Catalog No.:BCC6106
CAS No.:252979-43-4
- CHIR-98014
Catalog No.:BCC3751
CAS No.:252935-94-7
- CHIR-99021 (CT99021)
Catalog No.:BCC1275
CAS No.:252917-06-9
- TSU-68 (SU6668,Orantinib)
Catalog No.:BCC2508
CAS No.:252916-29-3
- RWJ 56110
Catalog No.:BCC7433
CAS No.:252889-88-6
- 20S,24R-Epoxydammar-12,25-diol-3-one
Catalog No.:BCN5118
CAS No.:25279-15-6
- Aristolene
Catalog No.:BCN8417
CAS No.:6831-16-9
- Apelin-36 (human)
Catalog No.:BCC5910
CAS No.:252642-12-9
- Ethyl orsellinate
Catalog No.:BCN4662
CAS No.:2524-37-0
- Lucidal
Catalog No.:BCN3206
CAS No.:252351-96-5
- Tetrabromobisphenol A diallyl ether
Catalog No.:BCC9174
CAS No.:25327-89-3
- Isocalamendiol
Catalog No.:BCN5119
CAS No.:25330-21-6
- BMS 195614
Catalog No.:BCC7740
CAS No.:253310-42-8
- Trazodone HCl
Catalog No.:BCC5032
CAS No.:25332-39-2
- Viniferin
Catalog No.:BCN3757
CAS No.:253435-07-3
- (+)-Corlumidine
Catalog No.:BCN2662
CAS No.:25344-54-1
- Wiskostatin
Catalog No.:BCC7934
CAS No.:253449-04-6
- 16-O-Acetylpolyporenic acid C
Catalog No.:BCN4058
CAS No.:2535-06-0
- 2-Amino-6-methylbenzothiazole
Catalog No.:BCC8543
CAS No.:2536-91-6
- Litseglutine B
Catalog No.:BCN5120
CAS No.:25368-01-8
- Asperulosidic acid
Catalog No.:BCN3088
CAS No.:25368-11-0
- TRIM
Catalog No.:BCC6847
CAS No.:25371-96-4
The effects of adriamycin (doxorubicin HCl) on human red blood cells.[Pubmed:6254923]
Hemoglobin. 1980;4(5-6):735-45.
We have studied the effects of adriamycin (doxorubicin HCl) on human red blood cells. The peroxidizing effect of adriamycin on the thiols of red cell constituents resulted in decreased glutathione stability, and oxidation of hemoglobin and membrane protein components 1, 2, and 3, forming large molecular weight complexes. Membrane lipids were also peroxidized. Adriamycin itself did not inhibit the enzymes of the reductions system (glucose-6-phosphate dehydrogenase, 6-phosphogluconic dehydrogenase, glutathione reductase, glutathione peroxidase, catalase, superoxide dismutase) of the red cells. Because adriamycin has the potential of inhibiting ATPase, including both Na-K-dependent ATPase and ouabain insensitive ATPase, at concentrations not inhibitory to other enzymes, the net sodium content increased, and potassium content decreased after incubation of red cells with adriamycin at high concentrations. The experimental results described with adriamycin may serve as a model for the possible mechanism of cardiotoxicity observed in its clinical use, and also explain the potential hemolyzing effect on red cells. There was greater oxidizing effect on glucose-6-phosphate dehydrogenase (G-6-PD) deficient than on normal erythrocytes. It is suggested that adriamycin be used with caution in individuals with G-6-PD deficient red cells.
In vitro release, pharmacokinetic and tissue distribution studies of doxorubicin hydrochloride (Adriamycin HCl) encapsulated in lipiodolized w/o emulsions and w/o/w multiple emulsions.[Pubmed:1409841]
Pharmazie. 1992 Jun;47(6):439-43.
The lipiodolized w/o emulsion or w/o/w multiple emulsion containing Doxorubicin hydrochloride (1; Adriamycin HCl) with different emulsifiers was prepared to evaluate in vitro sustained-release behavior, pharmacokinetic and tissue distribution function in Sprague Dawley (SD) rats. The results of dissolution indicate that the release of 1 was significantly sustained for both emulsions when HCO-60 (polyoxyethylene (60) hydrogenated castor oil) was used as an emulsifier. The serum concentration of 1 was reduced and prolonged for both emulsions with the increase of HCO-60. The C(max) level was lowered and T(max) value was delayed after administration of w/o emulsions with higher HCO-60 concentration. The apparent terminal half-life for 1 released from some emulsions with higher concentration of HCO-60 was 3-folds higher than that of the 1 solution. The clearance of some w/o or w/o/w ADR emulsions also decreased with the increase of HCO-60. Not only the concentration of 1 in heart and kidney decreased significantly after the administration of w/o emulsions with the higher concentration of HCO-60, but also the hepatic concentration of 1 was higher and increased with HCO-60 concentration. The hepatic 1 level became lower after administration of w/o/w multiple emulsions with the increase of HCO-60; however, the concentration of 1 in heart, lung and spleen increased somewhat. The results indicate that lipiodol and HCO-60 seemed to play an important role in the prolongation and selective retention of w/o emulsion or w/o/w multiple emulsion, in vitro and in vivo.
Pharmacologic reductions of total tau levels; implications for the role of microtubule dynamics in regulating tau expression.[Pubmed:16930453]
Mol Neurodegener. 2006 Jul 26;1:6.
UNLABELLED: The microtubule-associated protein tau (MAPT) is a pathological component of several neurodegenerative diseases and clinical dementias. Here, we have investigated the effects of a series of commercially available FDA-approved compounds and natural products on total tau protein levels using a cell-based approach that allows for the rapid and efficient measurement of changes in protein expression. RESULTS: The compounds that reduced tau largely fell within 3 functional categories with the largest percentage being microtubule regulators. Several of these candidates were validated in both a human neuroglioma and a human neuroblastoma cell line. While these drugs lead to a rapid reduction in tau protein levels, a selective decrease in MAPT mRNA expression was also observed. CONCLUSION: These findings suggest that the identified compounds that reduce tau levels may act either through direct effects on the MAPT promoter itself or by altering a feedback transcriptional mechanism regulating MAPT transcription. This is particularly interesting in light of recent evidence suggesting that MAPT 5' UTR mutations in late-onset PD and PSP cases alter the expression of tau mRNA. In fact, one of the compounds we identified, rotenone, has been used extensively to model PD in rodents. These observations may provide key insights into the mechanism of tau turnover within the neuron while also providing the first evidence that selectively reducing tau protein levels may be possible using compounds that are FDA-approved for other uses.
A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin.[Pubmed:10075079]
Biochem Pharmacol. 1999 Apr 1;57(7):727-41.
The mechanisms responsible for the antiproliferative and cytotoxic effects of the anthracycline antibiotics doxorubicin (Adriamycin) and daunorubicin (daunomycin) have been the subject of considerable controversy. This commentary addresses the potential role of DNA synthesis inhibition, free radical formation and lipid peroxidation, DNA binding and alkylation, DNA cross-linking, interference with DNA strand separation and helicase activity, direct membrane effects, and the initiation of DNA damage via the inhibition of topoisomerase II in the interaction of these drugs with the tumor cell. One premise underlying this analysis is that only studies utilizing drug concentrations that reflect the plasma levels in the patient after either bolus administration or continuous infusion are considered to reflect the basis for drug action in the clinic. The role of free radicals in anthracycline cardiotoxicity is also discussed.
Identification of yeast DNA topoisomerase II mutants resistant to the antitumor drug doxorubicin: implications for the mechanisms of doxorubicin action and cytotoxicity.[Pubmed:9380029]
Mol Pharmacol. 1997 Oct;52(4):658-66.
Doxorubicin is a therapeutically useful anticancer drug that exerts multiple biological effects. Its antitumor and cardiotoxic properties have been ascribed to anthracycline-mediated free radical damage to DNA and membranes. Evidence for this idea comes in part from the selection by doxorubicin from stationary phase yeast cells of mutants (petites) deficient in mitochondrial respiration and therefore defective in free radical generation. However, doxorubicin also binds to DNA topoisomerase II, converting the enzyme into a DNA damaging agent through the trapping of a covalent enzyme-DNA complex termed the 'cleavable complex.' We have used yeast to determine whether stabilization of cleavable complexes plays a role in doxorubicin action and cytotoxicity. A plasmid-borne yeast TOP2 gene was mutagenized with hydroxylamine and used to transform drug-permeable yeast strain JN394t2-4, which carries a temperature-sensitive top2-4 mutation in its chromosomal TOP2 gene. Selection in growth medium at the nonpermissive temperature of 35 degrees in the presence of doxorubicin resulted in the isolation of plasmid-borne top2 mutants specifying functional doxorubicin-resistant DNA topoisomerase II. Single-point changes of Gly748 to Glu or Ala642 to Ser in yeast topoisomerase II, which lie in and adjacent to the CAP-like DNA binding domain, respectively, were identified as responsible for resistance to doxorubicin, implicating these regions in drug action. None of the mutants selected in JN394t2-4, which has a rad52 defect in double-strand DNA break repair, was respiration-deficient. We conclude that topoisomerase II is an intracellular target for doxorubicin and that the genetic background and/or cell proliferation status can determine the relative importance of topoisomerase II- versus free radical-killing.
Studies on the in vitro reactivity of clofibryl and fenofibryl glucuronides. Evidence for protein binding via a Schiff's base mechanism.[Pubmed:8347161]
Biochem Pharmacol. 1993 Aug 3;46(3):357-64.
Clofibryl and fenofibryl acyl (ester) glucuronides (CAG and FAG) are major metabolites in humans of the hypolipidaemic drugs clofibrate and fenofibrate, respectively. We have investigated three inter-related aspects of the reactivity of CAG and FAG in human serum albumin (HSA) solution, human plasma and in buffer at pH 7.0: namely (a) rearrangement via acyl migration to glucuronic acid esters of clofibric acid (CA) and fenofibric acid (FA), (b) hydrolysis of the parent glucuronide and rearrangement products to yield CA and FA and (c) the formation of covalent adducts with albumin and plasma protein. CAG was more reactive than FAG in all media, especially the protein solutions. The reactivity of both glucuronides was accelerated in protein solution compared with buffer and this was more marked in plasma than in HSA solution. The predominant reaction during the initial stages of the incubation was formation of isomeric rearrangement products. In the protein solutions, CA and FA were the major reaction products after 24 hr, compared to the rearranged isomers in buffer. Protein binding of 14C to HSA was markedly higher after incubation of CAG and FAG labelled on the glucuronyl moiety compared with the label on the aglycone. This is consistent with the covalent binding of CAG and FAG to protein proceeding via the formation of a Schiff's base rather than by transacylation.


