TRIMCAS# 25371-96-4 |
- Gimeracil
Catalog No.:BCC2296
CAS No.:103766-25-2
- Mycophenolate Mofetil
Catalog No.:BCC2290
CAS No.:128794-94-5
- AGI-5198
Catalog No.:BCC2293
CAS No.:1355326-35-0
- AGI-6780
Catalog No.:BCC1331
CAS No.:1432660-47-3
- CPI-613
Catalog No.:BCC2287
CAS No.:95809-78-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 25371-96-4 | SDF | Download SDF |
| PubChem ID | 1359 | Appearance | Powder |
| Formula | C10H7F3N2 | M.Wt | 212.17 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in ethanol and to 50 mM in DMSO | ||
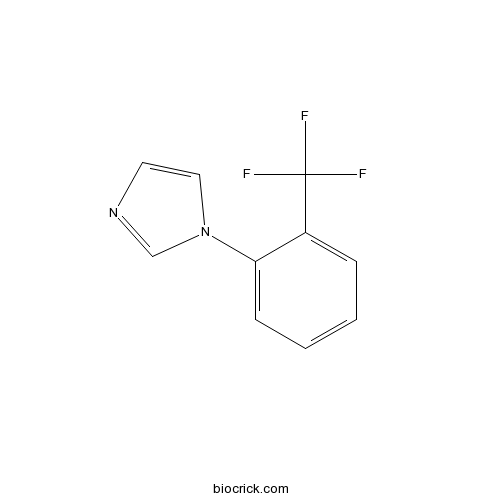
| Chemical Name | 1-[2-(trifluoromethyl)phenyl]imidazole | ||
| SMILES | C1=CC=C(C(=C1)C(F)(F)F)N2C=CN=C2 | ||
| Standard InChIKey | WZBWBNCQUTXYEL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H7F3N2/c11-10(12,13)8-3-1-2-4-9(8)15-6-5-14-7-15/h1-7H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A potent inhibitor of neuronal and inducible NO synthases, with much lower affinity for the endothelial isoform (displays IC50 values of 28.2, 27.0 and 1057.5 μM respectively). Antinociceptive in vivo. |

TRIM Dilution Calculator

TRIM Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.7132 mL | 23.566 mL | 47.132 mL | 94.264 mL | 117.83 mL |
| 5 mM | 0.9426 mL | 4.7132 mL | 9.4264 mL | 18.8528 mL | 23.566 mL |
| 10 mM | 0.4713 mL | 2.3566 mL | 4.7132 mL | 9.4264 mL | 11.783 mL |
| 50 mM | 0.0943 mL | 0.4713 mL | 0.9426 mL | 1.8853 mL | 2.3566 mL |
| 100 mM | 0.0471 mL | 0.2357 mL | 0.4713 mL | 0.9426 mL | 1.1783 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Asperulosidic acid
Catalog No.:BCN3088
CAS No.:25368-11-0
- Litseglutine B
Catalog No.:BCN5120
CAS No.:25368-01-8
- 2-Amino-6-methylbenzothiazole
Catalog No.:BCC8543
CAS No.:2536-91-6
- 16-O-Acetylpolyporenic acid C
Catalog No.:BCN4058
CAS No.:2535-06-0
- Wiskostatin
Catalog No.:BCC7934
CAS No.:253449-04-6
- (+)-Corlumidine
Catalog No.:BCN2662
CAS No.:25344-54-1
- Viniferin
Catalog No.:BCN3757
CAS No.:253435-07-3
- Trazodone HCl
Catalog No.:BCC5032
CAS No.:25332-39-2
- BMS 195614
Catalog No.:BCC7740
CAS No.:253310-42-8
- Isocalamendiol
Catalog No.:BCN5119
CAS No.:25330-21-6
- Tetrabromobisphenol A diallyl ether
Catalog No.:BCC9174
CAS No.:25327-89-3
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- AR-C 102222
Catalog No.:BCC6092
CAS No.:253771-21-0
- Kanamycin Sulfate
Catalog No.:BCC1205
CAS No.:25389-94-0
- Morroniside
Catalog No.:BCN5009
CAS No.:25406-64-8
- Boc-ß-HoAsp(OBzl)-OH
Catalog No.:BCC3229
CAS No.:254101-10-5
- Phellopterin
Catalog No.:BCN2637
CAS No.:2543-94-4
- H-Cys(pMeOBzl)-OH
Catalog No.:BCC2909
CAS No.:2544-31-2
- Dehydropitavastatin ethyl ester
Catalog No.:BCC8931
CAS No.:254452-91-0
- (+)-Afzelechin
Catalog No.:BCN5121
CAS No.:2545-00-8
- Felbamate
Catalog No.:BCC4904
CAS No.:25451-15-4
- H-D-Glu-OtBu
Catalog No.:BCC2938
CAS No.:25456-76-2
- H-Asn-OtBu
Catalog No.:BCC2877
CAS No.:25456-86-4
- Demethyleneberberine
Catalog No.:BCN2829
CAS No.:25459-91-0
Structural determinants of TRIM protein function.[Pubmed:28202672]
Biochem Soc Trans. 2017 Feb 8;45(1):183-191.
Tripartite motif (TRIM) proteins constitute one of the largest subfamilies of Really Interesting New Gene (RING) E3 ubiquitin ligases and contribute to the regulation of numerous cellular activities, including innate immune responses. The conserved TRIM harbours a RING domain that imparts E3 ligase activity to TRIM family proteins, whilst a variable C-terminal region can mediate recognition of substrate proteins. The knowledge of the structure of these multidomain proteins and the functional interplay between their constituent domains is paramount to understanding their cellular roles. To date, available structural information on TRIM proteins is still largely restricted to subdomains of many TRIMs in isolation. Nevertheless, applying a combination of structural, biophysical and biochemical approaches has recently allowed important progress to be made towards providing a better understanding of the molecular features that underlie the function of TRIM family proteins and has uncovered an unexpected diversity in the link between self-association and catalytic activity.
Evaluation of die trim morphology made by CAD-CAM technology.[Pubmed:28222879]
J Prosthet Dent. 2017 Sep;118(3):406-412.
STATEMENT OF PROBLEM: The die contour can affect the emergence profile of prosthetic restorations. However, little information is available regarding the congruency between a stereolithographic (SLA) die and its corresponding natural tooth. PURPOSE: The purpose of this vitro study was to evaluate the shapes of SLA die in comparison with the subgingival contour of a prepared tooth to be restored with a ceramic crown. MATERIAL AND METHODS: Twenty extracted human teeth, 10 incisors, and 10 molars, were disinfected and mounted in a typodont model. The teeth were prepared for a ceramic restoration. Definitive impressions were made using an intraoral scanner from which 20 SLA casts with removable dies were fabricated. The removable dies and corresponding human teeth were digitized using a 3-dimensional desktop scanner and evaluated with computer-aided design software. The subgingival morphology with regard to angle, length, and volume at the buccolingual and mesiodistal surfaces and at zones A, B, C, and D were compared. Data were first analyzed with repeated measures analysis of variance (ANOVA), using locations (buccolingual and mesiodistal), zones (A, B, C, and D), and model type (SLA and Natural) as within-subject factors and tooth type (molar and incisor) as the between-subject factor. Post hoc analyses were performed to investigate the difference between natural teeth and corresponding SLA models, depending upon the interaction effect from the repeated measures ANOVA (alpha=.05). RESULTS: For angle analysis, the incisor group demonstrated a significant difference between the natural tooth and SLA die on the buccolingual surfaces (P<.05), whereas the molar group demonstrated a significant difference at the mesiodistal surfaces (P<.05). For the evaluation of length and volume, the incisor group showed significant differences in zone D on both the buccolingual (P<.05) and the mesiodistal (P<.05) surfaces. However, significant differences in zones C (P<.05) and D (P<.05) on the buccolingual surfaces and in all zones on the mesiodistal surfaces were observed in the molar group. CONCLUSIONS: For the comparison of angles, SLA dies did not replicate the subgingival contour of natural teeth on the buccolingual surfaces of the incisal groups. For the comparison of length and volume, SLA dies were more concave and did not replicate the subgingival contour of natural teeth in the incisal and molar groups.
Expression profiling of TRIM protein family in THP1-derived macrophages following TLR stimulation.[Pubmed:28211536]
Sci Rep. 2017 Feb 17;7:42781.
Activated macrophages play an important role in many inflammatory diseases including septic shock and atherosclerosis. However, the molecular mechanisms limiting macrophage activation are not completely understood. Members of the tripartite motif (TRIM) family have recently emerged as important players in innate immunity and antivirus. Here, we systematically analyzed mRNA expressions of representative TRIM molecules in human THP1-derived macrophages activated by different toll-like receptor (TLR) ligands. Twenty-nine TRIM members were highly induced (>3 fold) by one or more TLR ligands, among which 19 of them belong to TRIM C-IV subgroup. Besides TRIM21, TRIM22 and TRIM38 were shown to be upregulated by TLR3 and TLR4 ligands as previous reported, we identified a novel group of TRIM genes (TRIM14, 15, 31, 34, 43, 48, 49, 51 and 61) that were significantly up-regulated by TLR3 and TLR4 ligands. In contrast, the expression of TRIM59 was down-regulated by TLR3 and TLR4 ligands in both human and mouse macrophages. The alternations of the TRIM proteins were confirmed by Western blot. Finally, overexpression of TRIM59 significantly suppressed LPS-induced macrophage activation, whereas siRNA-mediated knockdown of TRIM59 enhanced LPS-induced macrophage activation. Taken together, the study provided an insight into the TLR ligands-induced expressions of TRIM family in macrophages.
Nitric oxide mediates activity-dependent plasticity of retinal bipolar cell output via S-nitrosylation.[Pubmed:24305814]
J Neurosci. 2013 Dec 4;33(49):19176-93.
Coding a wide range of light intensities in natural scenes poses a challenge for the retina: adaptation to bright light should not compromise sensitivity to dim light. Here we report a novel form of activity-dependent synaptic plasticity, specifically, a "weighted potentiation" that selectively increases output of Mb-type bipolar cells in the goldfish retina in response to weak inputs but leaves the input-output ratio for strong stimuli unaffected. In retinal slice preparation, strong depolarization of bipolar terminals significantly lowered the threshold for calcium spike initiation, which originated from a shift in activation of voltage-gated calcium currents (ICa) to more negative potentials. The process depended upon glutamate-evoked retrograde nitric oxide (NO) signaling as it was eliminated by pretreatment with an NO synthase blocker, TRIM. The NO-dependent ICa modulation was cGMP independent but could be blocked by N-ethylmaleimide (NEM), indicating that NO acted via an S-nitrosylation mechanism. Importantly, the NO action resulted in a weighted potentiation of Mb output in response to small (/= 2.4 x 10(8) photons/cm(2)/s lowered the latency of scotopic (/= 3.5 x 10(9) photons/cm(2)/s) were unaltered. Under bright scotopic/mesopic conditions, this novel form of Mb output potentiation selectively amplifies dim retinal inputs at Mb --> ganglion cell synapses. We propose that this process might counteract decreases in retinal sensitivity during light adaptation by preventing the loss of visual information carried by dim scotopic signals.
Inhibition of nitric oxide synthase by 1-(2-trifluoromethylphenyl) imidazole (TRIM) in vitro: antinociceptive and cardiovascular effects.[Pubmed:8886430]
Br J Pharmacol. 1996 Sep;119(2):423-31.
1. The ability of a range of substituted imidazole compounds to inhibit mouse cerebellar neuronal nitric oxide synthase (nNOS), bovine aortic endothelial NOS (eNOS) and inducible NOS (iNOS) from lungs of endotoxin-pretreated rats was investigated. In each case the substrate (L-arginine) concentration employed was 120 nM. 2. 1-(2-Trifluoromethylphenyl) imidazole (TRIM) was a relatively potent inhibitor of nNOS and iNOS (IC50S of 28.2 microM and 27.0 microM respectively) but was a relatively weak inhibitor of eNOS (IC50, 1057.5 microM). The parent compound, imidazole, was a weak inhibitor of all three NOS isoforms (IC50S: nNOS, 290.6 microM; eNOS, 101.3 microM; iNOS, 616.0 microM). Substitution of imidazole with a phenyl group to yield I-phenylimidazole (PI) resulted in an isoform non-selective increase in inhibitory potency (IC50S: nNOS, 72.1 microM; eNOS, 86.9 microM; iNOS, 53.9 microM). Further substitution of the attached phenyl group resulted in an increase in nNOS and a decrease in eNOS inhibitory potency as in TRIM, 1-chlorophenylimidazole (CPI; IC50S: nNOS, 43.4 microM; eNOS, 392.3 microM; iNOS, 786.5 microM) and 1-(2,3,5,6-tetrafluorophenyl) imidazole (TETRA-FPI; IC50S; nNOS, 56.3 microM; eNOS, 559.6 microM; iNOS, 202.4 microM). 3. The ability of TRIM to inhibit mouse cerebellar nNOS activity in vitro was influenced by the concentration of L-arginine (0.12-10.0 microM) in the incubation medium. When mouse cerebellar nNOS was used as enzyme source a double reciprocal (Lineweaver-Burk) plot in the presence/absence of TRIM (50 microM) revealed a competitive inhibitory profile. The K(m) for L-arginine and the Ki for TRIM calculated from these data were 2.4 microM and 21.7 microM, respectively. The ability of TRIM to inhibit mouse cerebellar nNOS activity in vitro was unaffected by varying the time of exposure of the enzyme to TRIM from 0-60 min at 0 degree C. 4. TRIM exhibits potent antinociceptive activity in the mouse as evidenced by inhibition of acetic acid induced abdominal constrictions. The ED50 for TRIM following i.p. administration was 20 mg kg-1 (94.5 mumol kg-1). The antinociceptive effect of TRIM was reversed by pretreatment of animals with L-arginine (50 mg kg-1, i.p.) and was not accompanied by sedation, motor ataxia or behavioural changes (rearing, crossing, circling, dipping) as assessed by use of a box maze procedure. 5. L-NG nitro arginine methyl ester (L-NAME, 20 mg kg-1, i.v.) but not TRIM (0.5-20 mg kg-1, i.v.) increased mean arterial blood pressure (MAP) in the urethane-anaesthetized rat. 6. L-NAME (100 microM) potentiated the contractile response of the rabbit isolated aorta to phenylephrine (ED50; 0.084 +/- 0.01 microM in the presence and 0.25 +/- 0.05 microM in the absence of L-NAME; maximum response, 7.7 +/- 0.4 g in the presence and 5.6 +/- 0.5 g in the absence of L-NAME, n = 6, (P < 0.05) whilst TRIM (1-100 microM) was without effect. L-NAME (100 microM) but not TRIM (1-100 microM) also reduced carbachol-induced relaxation of the phenylephrine-precontracted rabbit aorta preparation. 7. L-NAME (50 microM) potentiated the vasoconstrictor effect of bolus-injected noradrenaline (10-1000 nmol) and reduced the vasodilator effect of carbachol (10 microM) added to the Krebs reservoir in the rat perfused mesentery preparation. L-NAME (50 microM) also reduced nitric oxide (NO) release (measured by chemiluminescence of nitrite in the Krebs perfusate) in response to noradrenaline (100 nmol; 53.8 +/- 4.0 pmol ml-1 in the presence and 84.8 +/- 8.0 pmol ml-1 in the absence of L-NAME, n = 15, P < 0.05) and carbachol (10 microM; 63.9 +/- 5.0 pmol ml-1 in the presence and 154.0 +/- 9.0 pmol ml-1 in the absence of L-NAME, n = 15, P < 0.05). TRIM (50 microM) did not affect either the vasoconstrictor response to noradrenaline or the vasodilator response to carbachol or the accompanying release of NO from the perfused rat mesentery.
The antinociceptive effect of 1-(2-trifluoromethylphenyl) imidazole (TRIM), a potent inhibitor of neuronal nitric oxide synthase in vitro, in the mouse.[Pubmed:8581267]
Br J Pharmacol. 1995 Nov;116(5):2349-50.
1-(2-trifluoromethylphenyl)imidazole (TRIM) is a potent inhibitor of neuronal (mouse cerebellar) and inducible (lung from endotoxin-pretreated rats) isoforms of nitric oxide synthase (NOS) with IC50 values of 28.2 microM and 27.0 microM, respectively. In contrast, TRIM is a poor inhibitor of bovine aortic endothelial NOS with an IC50 of 1057.5 microM. TRIM (10-50 mg kg-1) administered i.p. exhibits dose-related antinociceptive activity in the mouse (assessed as inhibition of late phase formalin-induced hindpaw licking behaviour) with an ED50 of 85.8 mumol kg-1. In contrast, TRIM (50 mg kg-1, i.p.) failed to influence mean arterial blood pressure in the urethane-anaesthetized mouse. Thus, TRIM may be of use as an experimental tool with which to investigate the biological roles of nitric oxide (NO) within the central nervous system.


