GimeracilDihydropyrimidine dehydrogenase inhibitor CAS# 103766-25-2 |
- Mycophenolate Mofetil
Catalog No.:BCC2290
CAS No.:128794-94-5
- AGI-5198
Catalog No.:BCC2293
CAS No.:1355326-35-0
- Trilostane
Catalog No.:BCC2302
CAS No.:13647-35-3
- AGI-6780
Catalog No.:BCC1331
CAS No.:1432660-47-3
- CPI-613
Catalog No.:BCC2287
CAS No.:95809-78-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 103766-25-2 | SDF | Download SDF |
| PubChem ID | 54679224 | Appearance | Powder |
| Formula | C5H4ClNO2 | M.Wt | 145.54 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Gimestat | ||
| Solubility | DMSO : 100 mg/mL (687.10 mM; Need ultrasonic) | ||
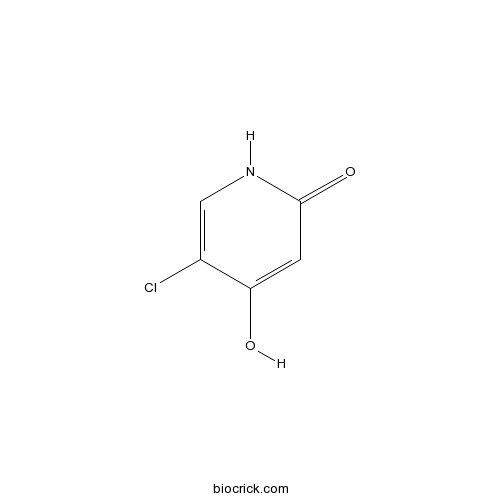
| Chemical Name | 5-chloro-4-hydroxy-1H-pyridin-2-one | ||
| SMILES | C1=C(C(=CNC1=O)Cl)O | ||
| Standard InChIKey | ZPLQIPFOCGIIHV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H4ClNO2/c6-3-2-7-5(9)1-4(3)8/h1-2H,(H2,7,8,9) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Gimeracil(Gimestat) is an inhibitor of dihydropyrimidine dehydrogenase (DPYD), which degrades pyrimidine including 5-fluorouracil in the blood; inhibits homologous recombination.
IC50 Value:
Target: DPYD
in vitro: Gimeracil had radiosensitizing effects by partially inhibiting homologous recombination (HR) in the repair of DNA double strand breaks. Tail moments in neutral comet assay increased in gimeracil-treated cells. Gimeracil restrained the formation of foci of Rad51 and replication protein A (RPA), whereas it increased the number of foci of Nbs1, Mre11, Rad50, and FancD2. Gimeracil did not sensitize DPYD-depleted cells [1]. Gimeracil inhibited DNA DSB repair. It did not sensitize cells deficient in HR but sensitized those deficient in NHEJ. In SCneo assay, Gimeracil reduced the frequency of neo-positive clones. Additionally, it sensitized the cells in S-phase more than in G0/G1 [2].
in vivo: References: | |||||

Gimeracil Dilution Calculator

Gimeracil Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.871 mL | 34.3548 mL | 68.7096 mL | 137.4193 mL | 171.7741 mL |
| 5 mM | 1.3742 mL | 6.871 mL | 13.7419 mL | 27.4839 mL | 34.3548 mL |
| 10 mM | 0.6871 mL | 3.4355 mL | 6.871 mL | 13.7419 mL | 17.1774 mL |
| 50 mM | 0.1374 mL | 0.6871 mL | 1.3742 mL | 2.7484 mL | 3.4355 mL |
| 100 mM | 0.0687 mL | 0.3435 mL | 0.6871 mL | 1.3742 mL | 1.7177 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Gimeracil is an inhibitor of dihydropyrimidine dehydrogenase, which inhibits the early step in homologous recombination for double strand breaks repair.
- R428
Catalog No.:BCC3692
CAS No.:1037624-75-1
- Shionone
Catalog No.:BCN1274
CAS No.:10376-48-4
- Fasudil
Catalog No.:BCC5262
CAS No.:103745-39-7
- Rehmaglutin D
Catalog No.:BCN5851
CAS No.:103744-84-9
- YK-4-279
Catalog No.:BCC2065
CAS No.:1037184-44-3
- Dihydrobonducellin
Catalog No.:BCN3731
CAS No.:103680-87-1
- Mirificin
Catalog No.:BCN2783
CAS No.:103654-50-8
- 2-Carbamoyl-3-hydroxy-1,4-naphthoquinone
Catalog No.:BCC8567
CAS No.:103646-20-4
- Ondansetron hydrochloride dihydrate
Catalog No.:BCC4213
CAS No.:103639-04-9
- Catechin 3-rhamnoside
Catalog No.:BCN5850
CAS No.:103630-03-1
- Cnidimol A
Catalog No.:BCN7167
CAS No.:103629-80-7
- Sumatriptan Succinate
Catalog No.:BCC2502
CAS No.:103628-48-4
- Ganoderic acid C2
Catalog No.:BCN3036
CAS No.:103773-62-2
- MBX-2982
Catalog No.:BCC1732
CAS No.:1037792-44-1
- KX2-391 dihydrochloride
Catalog No.:BCC1686
CAS No.:1038395-65-1
- 7-Prenylumbelliferone
Catalog No.:BCN2938
CAS No.:10387-50-5
- 4'-O-Methyllicoflavanone
Catalog No.:BCN4827
CAS No.:1038753-13-7
- Lazabemide hydrochloride
Catalog No.:BCC7371
CAS No.:103878-83-7
- Cycloart-25-ene-3,24-diol
Catalog No.:BCN5852
CAS No.:10388-48-4
- Procyanidin A1
Catalog No.:BCN6809
CAS No.:103883-03-0
- Lacidipine
Catalog No.:BCC4403
CAS No.:103890-78-4
- MK-4827
Catalog No.:BCC1761
CAS No.:1038915-60-4
- MK-4827 hydrochloride
Catalog No.:BCC4173
CAS No.:1038915-64-8
- MK-4827 tosylate
Catalog No.:BCC4174
CAS No.:1038915-73-9
Efficacy and safety of S-1 (tegafur, gimeracil, and oteracil potassium) concurrent with 3-dimensional conformal radiotherapy for newly diagnosed squamous cell carcinoma of the lung in elderly patients.[Pubmed:27068497]
Cancer Radiother. 2016 May;20(3):181-6.
PURPOSE: This study evaluated the efficacy and safety of the combination drug tegafur, Gimeracil, and oteracil potassium (S-1) concurrent with 3-dimensional conformal radiotherapy for newly diagnosed squamous cell carcinoma of the lung in elderly patients. PATIENTS AND METHODS: Patients with pathologically or cytologically newly diagnosed lung squamous cell carcinoma (n=106) were randomly assigned to receive the combination of tegafur, Gimeracil, and oteracil potassium (40mg/m(2), BID, d1-28, repeated every 6 weeks for 4 cycles) and concurrent 3D-conformal radiotherapy (60Gy; experimental group), or gemcitabine (800-1000mg/m(2), d1 and d8) repeated every 21 days for 4 cycles as well as 3D-conformal radiotherapy (control group). RESULTS: The overall response rate (complete and partial responses) of the experimental group was 68.6%, which was significantly higher than that of the control group (38.5%; P=0.002). The median progression-free survival rates of the experimental and control groups were 11.8 months (95% confidence interval [CI]: 8.0-22.4) and 7.8 months (95% CI, 6.9-9.2), respectively (P=0.017). Adverse reactions included grade I/II radiation esophagitis and pneumonitis, with good tolerance. Grade III/IV adverse reactions of the experimental and control groups were leucopenia (20% cf. 56.6%, respectively; P=0.027), thrombocytopenia (3.9% cf. 25%; P=0.037), and gastrointestinal reaction (1.9% cf. 3.5%; P=0.35). CONCLUSION: The efficacy of concurrent combination chemotherapy with tegafur, Gimeracil, and oteracil potassium (S-1) and 3D-conformal radiotherapy for newly diagnosed squamous cell carcinoma of the lung in elderly patients was excellent, and all toxicities were well tolerated. This treatment might be considered a main regimen in the management of squamous cell carcinoma of the lung in elderly patients.
Short-term clinical effect of conformal radiotherapy combined with tegafur gimeracil oteracil potassium in treating recurrent esophagus cancer.[Pubmed:27882010]
Pak J Med Sci. 2016 Sep-Oct;32(5):1141-1145.
OBJECTIVE: To observe clinical effects of three-dimensional conformal radiotherapy combined with Tegafur Gimeracil Oteracil Potassium chemotherapy in the treatment of patients with recurrent esophagus cancer. METHODS: One hundred and twelve senile patients who suffered from esophagus cancer were selected and randomly divided into two groups, namely, observation group (56 cases) and control group (56 cases). The observation group adopted three-dimensional conformal radiotherapy combined with Tegafur Gimeracil Oteracil Potassium chemotherapy and the control group adopted three-dimensional conformal radiotherapy only. RESULTS: All patients completed the treatment, with good compliance. Effective rate of the observation group was 82.1%, which was significantly higher than the control group (67.9%), and the difference was statistically significant (P<0.05). Main toxic and side effects of patients of two groups were radiation esophagitis, gastrointestinal reaction, hematologic toxicities and radiative skin reaction. Differences of incidence rates of all types of toxic and side effects were not statistically significant (P>0.05). The one-year and two-year survival rates of patients of the observation group were 80.4% and 53.6%, respectively, while the control group was 55.4% and 30.4%; differences between two groups were statistically significant (P<0.05). CONCLUSION: Three-dimensional conformal radiotherapy combined with Tegafur Gimeracil Oteracil Potassium chemotherapy has definite curative effect in treating patients with recurrent esophagus cancer and can improve survival rate of patients, without increasing adverse reaction.
Gimeracil Exerts Radiosensitizing Effects on Oral Squamous Cell Carcinoma Cells In Vitro and In Vivo.[Pubmed:27793917]
Anticancer Res. 2016 Nov;36(11):5923-5930.
BACKGROUND/AIM: Gimeracil or 5-chloro-2, 4-dihydroxypyridine (CDHP) has been reported to exert radiosensitization effects in cancer cells by suppressing DNA repair pathways. Here, we investigated the antitumor effect of Gimeracil and radiation combination therapy against oral squamous cell carcinoma (OSCC). MATERIAL AND METHODS: The antitumor activity of Gimeracil and/or radiation was investigated in HSC2 and/or SAS cells by growth inhibition assays and clonogenic survival assay. The expression of DNA double-strand break repair proteins were assessed by western blotting and immunohistochemistry, also fluorescent measurements of intracellular reactive oxygen/nitrogen species (ROS/RNS) were carried out in Gimeracil and/or radiation-treated HSC2 cells/tumors. RESULTS: Gimeracil and radiation combination treatment significantly inhibited OSCC cell/tumor growth and colony formation. Down-regulated expressions of DNA double-strand break repair proteins were observed in Gimeracil and/or radiation treated cells/tumors. Additionally, the growth inhibitory effect of this combination treatment was associated with reactive oxygen species/reactive nitrogen species (ROS/RNS) generation. CONCLUSION: Gimeracil might exert radiosensitizing effects on OSCC cells.


