MK-4827PARP-1/-2 inhibitor,potent and selective CAS# 1038915-60-4 |
- BMN-673 8R,9S
Catalog No.:BCC1422
CAS No.:1207456-00-5
- XAV-939
Catalog No.:BCC1120
CAS No.:284028-89-3
- PJ34
Catalog No.:BCC1865
CAS No.:344458-19-1
- ABT-888 (Veliparib)
Catalog No.:BCC1267
CAS No.:912444-00-9
- Veliparib dihydrochloride
Catalog No.:BCC2076
CAS No.:912445-05-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1038915-60-4 | SDF | Download SDF |
| PubChem ID | 24958200 | Appearance | Powder |
| Formula | C19H20N4O | M.Wt | 320.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Niraparib | ||
| Solubility | DMSO : 25 mg/mL (78.03 mM; Need ultrasonic) | ||
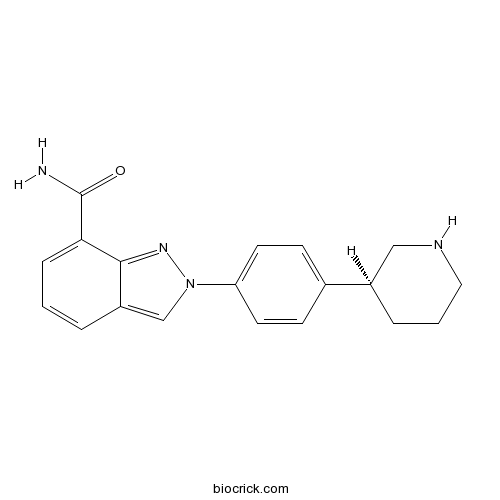
| Chemical Name | 2-[4-[(3S)-piperidin-3-yl]phenyl]indazole-7-carboxamide | ||
| SMILES | C1CC(CNC1)C2=CC=C(C=C2)N3C=C4C=CC=C(C4=N3)C(=O)N | ||
| Standard InChIKey | PCHKPVIQAHNQLW-CQSZACIVSA-N | ||
| Standard InChI | InChI=1S/C19H20N4O/c20-19(24)17-5-1-3-15-12-23(22-18(15)17)16-8-6-13(7-9-16)14-4-2-10-21-11-14/h1,3,5-9,12,14,21H,2,4,10-11H2,(H2,20,24)/t14-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | MK-4827 is a potent, selective inhibitor of PARP 1 and PARP 2 with IC50 of 3.8 and 2.1 nM, respectively. | |||||
| Targets | PARP1 | PARP2 | ||||
| IC50 | 3.8 nM | 2.1 nM | ||||

MK-4827 Dilution Calculator

MK-4827 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1212 mL | 15.606 mL | 31.212 mL | 62.4239 mL | 78.0299 mL |
| 5 mM | 0.6242 mL | 3.1212 mL | 6.2424 mL | 12.4848 mL | 15.606 mL |
| 10 mM | 0.3121 mL | 1.5606 mL | 3.1212 mL | 6.2424 mL | 7.803 mL |
| 50 mM | 0.0624 mL | 0.3121 mL | 0.6242 mL | 1.2485 mL | 1.5606 mL |
| 100 mM | 0.0312 mL | 0.1561 mL | 0.3121 mL | 0.6242 mL | 0.7803 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
MK-4827 is a potent, selective, PARP 1/2 inhibitor with IC50 of 3.8 and 2.1 nM for PARP1 and 2, respectively. MK-4827 possesses potential antineoplastic activity. In a whole cell assay, MK-4827 prevented PARP activity with an EC50 of 4 nM, enhancing the accumulation of DNA strand breaks and promoting genomic instability and apoptosis. MK-4827 induces selective synthetic lethality in homologous recombination (HR) repair deficient tumors with BRCA1/2 loss and tumor cell lines with non-BRCA-related HR defects, supporting clinical utility in sporadic tumors. MK-4827 reveals good pharmacokinetic properties and is currently in phase I clin. trials. The phase I clinical trials for MK-4827 is ongoing in the treatment of solid tumors.
- Lacidipine
Catalog No.:BCC4403
CAS No.:103890-78-4
- Procyanidin A1
Catalog No.:BCN6809
CAS No.:103883-03-0
- Cycloart-25-ene-3,24-diol
Catalog No.:BCN5852
CAS No.:10388-48-4
- Lazabemide hydrochloride
Catalog No.:BCC7371
CAS No.:103878-83-7
- 4'-O-Methyllicoflavanone
Catalog No.:BCN4827
CAS No.:1038753-13-7
- 7-Prenylumbelliferone
Catalog No.:BCN2938
CAS No.:10387-50-5
- KX2-391 dihydrochloride
Catalog No.:BCC1686
CAS No.:1038395-65-1
- MBX-2982
Catalog No.:BCC1732
CAS No.:1037792-44-1
- Ganoderic acid C2
Catalog No.:BCN3036
CAS No.:103773-62-2
- Gimeracil
Catalog No.:BCC2296
CAS No.:103766-25-2
- R428
Catalog No.:BCC3692
CAS No.:1037624-75-1
- Shionone
Catalog No.:BCN1274
CAS No.:10376-48-4
- MK-4827 hydrochloride
Catalog No.:BCC4173
CAS No.:1038915-64-8
- MK-4827 tosylate
Catalog No.:BCC4174
CAS No.:1038915-73-9
- MK-4827 Racemate
Catalog No.:BCC5179
CAS No.:1038915-75-1
- Mannioside A
Catalog No.:BCN5853
CAS No.:1038922-95-0
- 17β-Hydroxy-17-methylandrosta-4,9(11)-dien-3-one
Catalog No.:BCC8444
CAS No.:1039-17-4
- Maxacalcitol
Catalog No.:BCC1730
CAS No.:103909-75-7
- (-)-Isodocarpin
Catalog No.:BCN3280
CAS No.:10391-08-9
- Nodosin
Catalog No.:BCN5854
CAS No.:10391-09-0
- Lupeol caffeate
Catalog No.:BCN5855
CAS No.:103917-26-6
- Oleanolic acid 3-O-beta-D-glucosyl-(1->3)-alpha-L-rhamnosyl(1->2)-alpha-L-arabinoside
Catalog No.:BCN8132
CAS No.:103956-33-8
- 15-Nor-14-oxolabda-8(17),12-dien-18-oic acid
Catalog No.:BCN1637
CAS No.:1039673-32-9
- Esculentic acid
Catalog No.:BCN5856
CAS No.:103974-74-9
Discovery of 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide (MK-4827): a novel oral poly(ADP-ribose)polymerase (PARP) inhibitor efficacious in BRCA-1 and -2 mutant tumors.[Pubmed:19873981]
J Med Chem. 2009 Nov 26;52(22):7170-85.
We disclose the development of a novel series of 2-phenyl-2H-indazole-7-carboxamides as poly(ADP-ribose)polymerase (PARP) 1 and 2 inhibitors. This series was optimized to improve enzyme and cellular activity, and the resulting PARP inhibitors display antiproliferation activities against BRCA-1 and BRCA-2 deficient cancer cells, with high selectivity over BRCA proficient cells. Extrahepatic oxidation by CYP450 1A1 and 1A2 was identified as a metabolic concern, and strategies to improve pharmacokinetic properties are reported. These efforts culminated in the identification of 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide 56 (MK-4827), which displays good pharmacokinetic properties and is currently in phase I clinical trials. This compound displays excellent PARP 1 and 2 inhibition with IC(50) = 3.8 and 2.1 nM, respectively, and in a whole cell assay, it inhibited PARP activity with EC(50) = 4 nM and inhibited proliferation of cancer cells with mutant BRCA-1 and BRCA-2 with CC(50) in the 10-100 nM range. Compound 56 was well tolerated in vivo and demonstrated efficacy as a single agent in a xenograft model of BRCA-1 deficient cancer.
Niraparib (MK-4827), a novel poly(ADP-Ribose) polymerase inhibitor, radiosensitizes human lung and breast cancer cells.[Pubmed:24970803]
Oncotarget. 2014 Jul 15;5(13):5076-86.
The aim of this study was to assess niraparib (MK-4827), a novel poly(ADP-Ribose) polymerase (PARP) inhibitor, for its ability to radiosensitize human tumor cells. Human tumor cells derived from lung, breast and prostate cancers were tested for radiosensitization by niraparib using clonogenic survival assays. Both p53 wild-type and p53-defective lines were included. The ability of niraparib to alter the repair of radiation-induced DNA double strand breaks (DSBs) was determined using detection of gamma-H2AX foci and RAD51 foci. Clonogenic survival analyses indicated that micromolar concentrations of niraparib radiosensitized tumor cell lines derived from lung, breast, and prostate cancers independently of their p53 status but not cell lines derived from normal tissues. Niraparib also sensitized tumor cells to H2O2 and converted H2O2-induced single strand breaks (SSBs) into DSBs during DNA replication. These results indicate that human tumor cells are significantly radiosensitized by the potent and selective PARP-1 inhibitor, niraparib, in the in vitro setting. The mechanism of this effect appears to involve a conversion of sublethal SSBs into lethal DSBs during DNA replication due to the inhibition of base excision repair by the drug. Taken together, our findings strongly support the clinical evaluation of niraparib in combination with radiation.
Poly (ADP-Ribose) polymerase inhibitor MK-4827 together with radiation as a novel therapy for metastatic neuroblastoma.[Pubmed:23482742]
Anticancer Res. 2013 Mar;33(3):755-62.
BACKGROUND/AIM: To assess poly (ADP-ribose) polymerase (PARP) inhibitor MK-4827 together with radiation for the treatment of neuroblastoma. MATERIALS AND METHODS: Clonogenic survival assays were used to assess MK-4827, radiation and combination thereof in four neuroblastoma cell lines. In vivo efficacy was tested in a murine xenograft model of metastatic neuroblastoma. In vivo targeted inhibition and biological effects included measurement of cleaved caspase-3, gamma-H2AX, and Ki 67 by immunohistochemistry (IHC) and poly-ADP-ribose by Enzyme-Linked Immunosorbent Assay. RESULTS: Treatment of neuroblastoma cell lines reduced clonogenicity and resulted in additive effects with radiation. In vivo treatment with MK-4827 and radiation prolonged survival (p<0.01) compared to single modalities. In vivo superiority of MK-4827 plus radiation was further documented by significant elevations of cleaved caspase-3 and gamma-H2AX in tumors from the combination group compared to single modality cohorts. CONCLUSION: Combination of MK-4827 and radiation might provide effective therapy for children with high-risk neuroblastoma.
MK-4827, a PARP-1/-2 inhibitor, strongly enhances response of human lung and breast cancer xenografts to radiation.[Pubmed:22127459]
Invest New Drugs. 2012 Dec;30(6):2113-20.
The poly-(ADP-ribose) polymerase (PARP) inhibitor, MK-4827, is a novel potent, orally bioavailable PARP-1 and PARP-2 inhibitor currently in phase I clinical trials for cancer treatment. No preclinical data currently exist on the combination of MK-4827 with radiotherapy. The current study examined combined treatment efficacy of MK-4827 and fractionated radiotherapy using a variety of human tumor xenografts of differing p53 status: Calu-6 (p53 null), A549 (p53 wild-type [wt]) and H-460 (p53 wt) lung cancers and triple negative MDA-MB-231 human breast carcinoma. To mimic clinical application of radiotherapy, fractionated radiation (2 Gy per fraction) schedules given once or twice daily for 1 to 2 weeks combined with MK-4827, 50 mg/kg once daily or 25 mg/kg twice daily, were used. MK-4827 was found to be highly and similarly effective in both radiation schedules but maximum radiation enhancement was observed when MK-4827 was given at a dose of 50 mg/kg once daily (EF = 2.2). MK-4827 radiosensitized all four tumors studied regardless of their p53 status. MK-4827 reduced PAR levels in tumors by 1 h after administration which persisted for up to 24 h. This long period of PARP inhibition potentially adds to the flexibility of design of future clinical trials. Thus, MK-4827 shows high potential to improve the efficacy of radiotherapy.


