BMN-673 8R,9SPARP inhibitor CAS# 1207456-00-5 |
- LY2606368
Catalog No.:BCC4105
CAS No.:1234015-52-1
- AZD7762
Catalog No.:BCC2555
CAS No.:860352-01-8
- MK-8776 (SCH-900776)
Catalog No.:BCC3817
CAS No.:891494-63-6
- LY2603618
Catalog No.:BCC3923
CAS No.:911222-45-2
- PF-477736
Catalog No.:BCC4421
CAS No.:952021-60-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1207456-00-5 | SDF | Download SDF |
| PubChem ID | 44819242 | Appearance | Powder |
| Formula | C19H14F2N6O | M.Wt | 380.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Talazoparib (8R,9S); (8R,9S)-LT-673 | ||
| Solubility | DMSO : ≥ 50 mg/mL (131.46 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
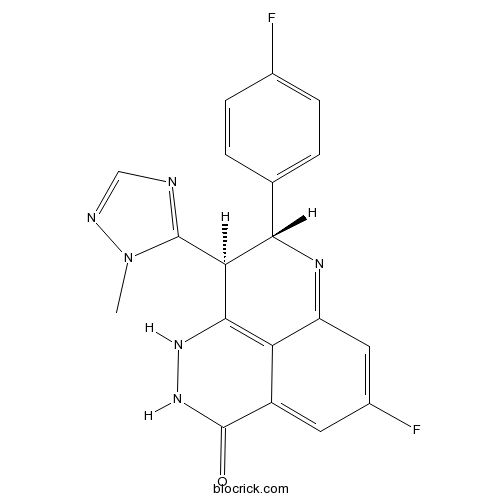
| SMILES | CN1C(=NC=N1)C2C(N=C3C=C(C=C4C3=C2NNC4=O)F)C5=CC=C(C=C5)F | ||
| Standard InChIKey | IUEWAGVJRJORLA-HOTGVXAUSA-N | ||
| Standard InChI | InChI=1S/C19H14F2N6O/c1-27-18(22-8-23-27)15-16(9-2-4-10(20)5-3-9)24-13-7-11(21)6-12-14(13)17(15)25-26-19(12)28/h2-8,15-16,25H,1H3,(H,26,28)/t15-,16-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | BMN 673 is a novel PARP inhibitor with IC50 of 0.58 nM(PARP1). It does not inhibit PARG and is highly sensitive to PTEN mutation. | |||||
| Targets | PARP | |||||
| IC50 | 0.58 nM | |||||

BMN-673 8R,9S Dilution Calculator

BMN-673 8R,9S Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6292 mL | 13.1458 mL | 26.2916 mL | 52.5831 mL | 65.7289 mL |
| 5 mM | 0.5258 mL | 2.6292 mL | 5.2583 mL | 10.5166 mL | 13.1458 mL |
| 10 mM | 0.2629 mL | 1.3146 mL | 2.6292 mL | 5.2583 mL | 6.5729 mL |
| 50 mM | 0.0526 mL | 0.2629 mL | 0.5258 mL | 1.0517 mL | 1.3146 mL |
| 100 mM | 0.0263 mL | 0.1315 mL | 0.2629 mL | 0.5258 mL | 0.6573 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PARP1/2 inhibitors are a class of anticancer agents that target tumor-specific defects in DNA repair. BMN 673 is a highly potent PARP1/2 inhibitor.
In vitro: BMN 673 is a potent PARP1/2 inhibitor, but it does not inhibit other enzymes that we have tested. BMN673 exhibits selective antitumor cytotoxicity and elicits DNA repair biomarkers at much lower concentrations than earlier generation PARP1/2 inhibitors. BMN 673 targeted tumor cells with BRCA1, BRCA2, or PTEN gene defects selectively with 20- to more than 200-fold greater potency than existing PARP1/2 inhibitors in vitro [1].
In vivo: BMN 673 is readily orally bioavailable, with more than 40% absolute oral bioavailability in rats when dosed in CMC. Orally BMN 673 elicited remarkable antitumor activity in mice; xenografted tumors that carry defects in DNA repair due to BRCA mutations or PTEN deficiency were profoundly sensitive to oral BMN 673 treatment at well-tolerated doses. Synergistic antitumor effects were also found when BMN 673 was combined with temozolomide, SN38, or platinum drugs [1].
Clinical trial: Pharmacokinetics (PK), pharmacodynamics (PD), safety and anti-tumor activity of BMN 673 were evaluated in a 2-stage dose-escalation study with 3-6 patients (pts)/dose level. Results showed BMN 673 was well tolerated with impressive anti-tumor activity in pts with BRCA mut with a single agent recommended Phase II trial dose of 1000 μg/d due to dose-limiting thrombocytopenia.
Reference:
[1] Shen Y, Rehman FL, Feng Y, Boshuizen J, Bajrami I, Elliott R, Wang B, Lord CJ, Post LE, Ashworth A. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin Cancer Res. 2013;19(18):5003-15.
- GDC-0349
Catalog No.:BCC1094
CAS No.:1207360-89-1
- GDC-mTOR inhibitor
Catalog No.:BCC1781
CAS No.:1207358-59-5
- BI-847325
Catalog No.:BCC6511
CAS No.:1207293-36-4
- NVS-CRF38
Catalog No.:BCC8059
CAS No.:1207258-55-6
- 12alpha-Hydroxyevodol
Catalog No.:BCN6102
CAS No.:120722-04-5
- Sarcandrolide D
Catalog No.:BCN6621
CAS No.:1207185-03-2
- Scutebata G
Catalog No.:BCN6101
CAS No.:1207181-63-2
- Scutebata F
Catalog No.:BCN6100
CAS No.:1207181-62-1
- Scutebata E
Catalog No.:BCN6099
CAS No.:1207181-61-0
- Scutebata C
Catalog No.:BCN6098
CAS No.:1207181-59-6
- Scutebata B
Catalog No.:BCN6097
CAS No.:1207181-58-5
- Scutebata A
Catalog No.:BCN6096
CAS No.:1207181-57-4
- BMN 673
Catalog No.:BCC2205
CAS No.:1207456-01-6
- 5-OMe-UDP trisodium salt
Catalog No.:BCC6153
CAS No.:1207530-98-0
- LDV FITC
Catalog No.:BCC6229
CAS No.:1207610-07-8
- 3,2'-Epilarixinol
Catalog No.:BCN6496
CAS No.:1207671-28-0
- Huperzine A
Catalog No.:BCN1058
CAS No.:120786-18-7
- Gynosaponin I
Catalog No.:BCN4078
CAS No.:1207861-69-5
- Quassidine B
Catalog No.:BCN7022
CAS No.:1207862-37-0
- CaMKII-IN-1
Catalog No.:BCC5530
CAS No.:1208123-85-6
- VU 0365114
Catalog No.:BCC6164
CAS No.:1208222-39-2
- Ketone Ester
Catalog No.:BCC1677
CAS No.:1208313-97-6
- N6022
Catalog No.:BCC4127
CAS No.:1208315-24-5
- PF-03394197(Oclacitinib)
Catalog No.:BCC6474
CAS No.:1208319-26-9


