BMN 673Potent PARP inhibitor CAS# 1207456-01-6 |
- MK-4827
Catalog No.:BCC1761
CAS No.:1038915-60-4
- Iniparib (BSI-201)
Catalog No.:BCC2208
CAS No.:160003-66-7
- AG-14361
Catalog No.:BCC2209
CAS No.:328543-09-5
- PJ34 hydrochloride
Catalog No.:BCC2210
CAS No.:344458-15-7
- Rucaparib (AG-014699,PF-01367338)
Catalog No.:BCC2207
CAS No.:459868-92-9
- Veliparib dihydrochloride
Catalog No.:BCC2076
CAS No.:912445-05-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1207456-01-6 | SDF | Download SDF |
| PubChem ID | 44819241 | Appearance | Powder |
| Formula | C19H14F2N6O | M.Wt | 380.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Talazoparib; LT-673 | ||
| Solubility | DMSO : 33.33 mg/mL (87.63 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
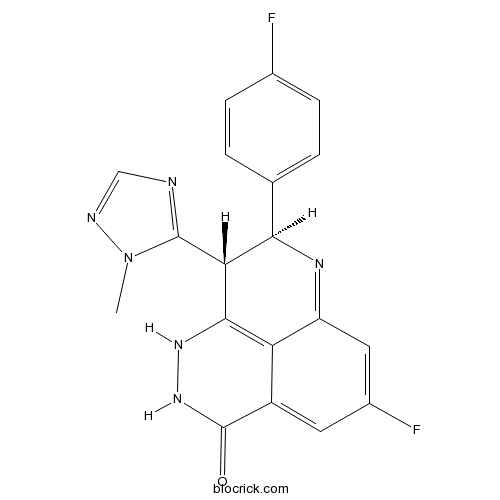
| SMILES | CN1C(=NC=N1)C2C(N=C3C=C(C=C4C3=C2NNC4=O)F)C5=CC=C(C=C5)F | ||
| Standard InChIKey | IUEWAGVJRJORLA-HZPDHXFCSA-N | ||
| Standard InChI | InChI=1S/C19H14F2N6O/c1-27-18(22-8-23-27)15-16(9-2-4-10(20)5-3-9)24-13-7-11(21)6-12-14(13)17(15)25-26-19(12)28/h2-8,15-16,25H,1H3,(H,26,28)/t15-,16-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | BMN673 is a potent and selective inhibitor of PARP1,PARP2 with Ki of 1.2 and 0.9 nM, respectively . | |||||
| Targets | PARP | |||||
| IC50 | 0.58 nM | |||||
| Kinase experiment [1]: | |
| PARP enzyme assays | For PARP inhibitor Ki determination, enzyme assays were conducted in 96-well FlashPlate with 0.5 U PARP1 enzyme , 0.25x activated DNA, 0.2 mCi [3H] NAD, and 5 mmol/L cold NAD(Sigma) in a final volume of 50 mL reaction buffer containing 10% glycerol (v/v), 25 mmol/L HEPES, 12.5 mmol/L MgCl2, 50 mmol/L KCl, 1 mmol/L dithiothreitol (DTT), and 0.01% NP-40 (v/v), pH 7.6. Reactions were initiated by adding NAD to the PARP reaction mixture with or without inhibitors and incubated for 1 minute at room temperature. Fifty microliter of ice-cold 20% trichloroacetic acid (TCA) was then added to each well to stop the reaction. The plate was sealed and shaken for a further 120 minutes at room temperature, followed by centrifugation. Radioactive signal bound to the FlashPlate was determined using Top-Count. PARP1 Km was determined using Michaelis–Menten equation from various substrate concentrations (1–100 mmol/L NAD). Compound Ki was calculated from enzyme inhibition curve according to the formula: Ki ¼ IC50/[1þ (substrate)/Km]. Km for PARP2 enzyme and compound Ki were determined with the same assay protocol except 30 ng PARP2, 0.25x activated DNA, 0.2 mCi [3H] NAD, and 20 mmol/L cold NAD were used in the reaction for 30 minutes at room temperature. |
| Cell experiment [2]: | |
| Cell lines | SCLC cell lines |
| Preparation method | Limited solubility. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
| Reacting condition | 24 h-96 h |
| Applications | BMN 673 exhibits a potent inhibitory effect on a panel of 11 SCLC cell lines (IC50=1.7 to 15 nmol/L), which are all within clinically achievable ranges. In addition, sensitivity to BMN673 correlates to DNA repair protein expression and PI3K pathway activity. |
| Animal experiment [1]: | |
| Animal models | Nude mice bearing established subcutaneous MX-1 tumor xenografts. |
| Dosage form | Oral gavage and twice daily for 28 consecutive days. |
| Application | BMN 673 inhibits the growth of MX-1 xenografts in mice, with 4 of 6 mice achieving a complete response. 0.33 and 0.1 mg/kg BMN 673 is well tolerated, with no animal lethality. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: 1. Shen Y, Rehman FL, Feng Y et al. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin Cancer Res. 2013 Sep 15;19(18):5003-15. 2. Cardnell RJ, Feng Y, Diao L et al. Proteomic markers of DNA repair and PI3K pathway activation predict response to the PARP inhibitor BMN 673 in small cell lung cancer. Clin Cancer Res. 2013 Nov 15;19(22):6322-8. | |

BMN 673 Dilution Calculator

BMN 673 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6292 mL | 13.1458 mL | 26.2916 mL | 52.5831 mL | 65.7289 mL |
| 5 mM | 0.5258 mL | 2.6292 mL | 5.2583 mL | 10.5166 mL | 13.1458 mL |
| 10 mM | 0.2629 mL | 1.3146 mL | 2.6292 mL | 5.2583 mL | 6.5729 mL |
| 50 mM | 0.0526 mL | 0.2629 mL | 0.5258 mL | 1.0517 mL | 1.3146 mL |
| 100 mM | 0.0263 mL | 0.1315 mL | 0.2629 mL | 0.5258 mL | 0.6573 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BMN673 is a potent and selective PARP1/2 inhibitor with Ki of 1.2 and 0.9 nM, respectively 1. It had no effect on panels of 72 receptors, ion channels, and enzymes 1. BMN673 showed IC50 value of 0.57 nM in enzymatic assay of PARP1 1. In in vitro assay, it exhibited greater potency than other existing PARP inhibitors, such as veliparib, rucaparib, and olaparib 2. It is also much more potent at trapping PARP-DNA complexes than other PARP inhibitors 3.
BMN673 has shown anti-tumor activity both in vitro and in vivo. It inhibited proliferation of tumor cells and xenografts with defects in homologous recombination 1. The combination of BMN673 and DNA-damaging agents demonstrated synergistic anti-tumor effects 1. In addition, study showed that the expression levels of DNA repair proteins and status of PI3K pathway predict response to BMN673 in small cell lung cancer 4.
BMN673 is currently under investigation in multiple clinical trials for advanced solid tumors or hematological malignancies, either as monotherapy or in combination with other anti-tumor agents.
References:
1. Shen Y, Rehman FL, Feng Y et al. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin Cancer Res 2013; 19: 5003-5015.
2. Cardnell RJ, Byers LA. Proteomic Markers of DNA Repair and PI3K Pathway Activation Predict Response to the PARP Inhibitor BMN 673 in Small Cell Lung Cancer--Response. Clin Cancer Res 2014; 20: 2237.
3. Murai J, Huang SY, Renaud A et al. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther 2014; 13: 433-443.
4. Cardnell RJ, Feng Y, Diao L et al. Proteomic markers of DNA repair and PI3K pathway activation predict response to the PARP inhibitor BMN 673 in small cell lung cancer. Clin Cancer Res 2013; 19: 6322-6328.
- BMN-673 8R,9S
Catalog No.:BCC1422
CAS No.:1207456-00-5
- GDC-0349
Catalog No.:BCC1094
CAS No.:1207360-89-1
- GDC-mTOR inhibitor
Catalog No.:BCC1781
CAS No.:1207358-59-5
- BI-847325
Catalog No.:BCC6511
CAS No.:1207293-36-4
- NVS-CRF38
Catalog No.:BCC8059
CAS No.:1207258-55-6
- 12alpha-Hydroxyevodol
Catalog No.:BCN6102
CAS No.:120722-04-5
- Sarcandrolide D
Catalog No.:BCN6621
CAS No.:1207185-03-2
- Scutebata G
Catalog No.:BCN6101
CAS No.:1207181-63-2
- Scutebata F
Catalog No.:BCN6100
CAS No.:1207181-62-1
- Scutebata E
Catalog No.:BCN6099
CAS No.:1207181-61-0
- Scutebata C
Catalog No.:BCN6098
CAS No.:1207181-59-6
- Scutebata B
Catalog No.:BCN6097
CAS No.:1207181-58-5
- 5-OMe-UDP trisodium salt
Catalog No.:BCC6153
CAS No.:1207530-98-0
- LDV FITC
Catalog No.:BCC6229
CAS No.:1207610-07-8
- 3,2'-Epilarixinol
Catalog No.:BCN6496
CAS No.:1207671-28-0
- Huperzine A
Catalog No.:BCN1058
CAS No.:120786-18-7
- Gynosaponin I
Catalog No.:BCN4078
CAS No.:1207861-69-5
- Quassidine B
Catalog No.:BCN7022
CAS No.:1207862-37-0
- CaMKII-IN-1
Catalog No.:BCC5530
CAS No.:1208123-85-6
- VU 0365114
Catalog No.:BCC6164
CAS No.:1208222-39-2
- Ketone Ester
Catalog No.:BCC1677
CAS No.:1208313-97-6
- N6022
Catalog No.:BCC4127
CAS No.:1208315-24-5
- PF-03394197(Oclacitinib)
Catalog No.:BCC6474
CAS No.:1208319-26-9
- Isoliquiritin apioside
Catalog No.:BCN2914
CAS No.:120926-46-7
PARP1 expression, activity and ex vivo sensitivity to the PARP inhibitor, talazoparib (BMN 673), in chronic lymphocytic leukaemia.[Pubmed:26539646]
Oncotarget. 2015 Dec 22;6(41):43978-91.
In chronic lymphocytic leukemia (CLL), mutation and loss of p53 and ATM abrogate DNA damage signalling and predict poorer response and shorter survival. We hypothesised that poly (ADP-ribose) polymerase (PARP) activity, which is crucial for repair of DNA breaks induced by oxidative stress or chemotherapy, may be an additional predictive biomarker and a target for therapy with PARP inhibitors.We measured PARP activity in 109 patient-derived CLL samples, which varied widely (192 - 190052 pmol PAR/10(6) cells) compared to that seen in healthy volunteer lymphocytes (2451 - 7519 pmol PAR/10(6) cells). PARP activity was associated with PARP1 protein expression and endogenous PAR levels. PARP activity was not associated with p53 or ATM loss, Binet stage, IGHV mutational status or survival, but correlated with Bcl-2 and Rel A (an NF-kB subunit). Levels of 8-hydroxy-2'-deoxyguanosine in DNA (a marker of oxidative damage) were not associated with PAR levels or PARP activity. The potent PARP inhibitor, talazoparib (BMN 673), inhibited CD40L-stimulated proliferation of CLL cells at nM concentrations, independently of Binet stage or p53/ATM function.PARP activity is highly variable in CLL and correlates with stress-induced proteins. Proliferating CLL cells (including those with p53 or ATM loss) are highly sensitive to the PARP inhibitor talazoparib.
Discovery and Characterization of (8S,9R)-5-Fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-2,7,8,9-te trahydro-3H-pyrido[4,3,2-de]phthalazin-3-one (BMN 673, Talazoparib), a Novel, Highly Potent, and Orally Efficacious Poly(ADP-ribose) Polymerase-1/2 Inhibitor, as an Anticancer Agent.[Pubmed:26652717]
J Med Chem. 2016 Jan 14;59(1):335-57.
We discovered and developed a novel series of tetrahydropyridophthlazinones as poly(ADP-ribose) polymerase (PARP) 1 and 2 inhibitors. Lead optimization led to the identification of (8S,9R)-47 (talazoparib; BMN 673; (8S,9R)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-2,7,8,9-te trahydro-3H-pyrido[4,3,2-de]phthalazin-3-one). The novel stereospecific dual chiral-center-embedded structure of this compound has enabled extensive and unique binding interactions with PARP1/2 proteins. (8S,9R)-47 demonstrates excellent potency, inhibiting PARP1 and PARP2 enzyme activity with Ki = 1.2 and 0.87 nM, respectively. It inhibits PARP-mediated PARylation in a whole-cell assay with an EC50 of 2.51 nM and prevents proliferation of cancer cells carrying mutant BRCA1/2, with EC50 = 0.3 nM (MX-1) and 5 nM (Capan-1), respectively. (8S,9R)-47 is orally available, displaying favorable pharmacokinetic (PK) properties and remarkable antitumor efficacy in the BRCA1 mutant MX-1 breast cancer xenograft model following oral administration as a single-agent or in combination with chemotherapy agents such as temozolomide and cisplatin. (8S,9R)-47 has completed phase 1 clinical trial and is currently being studied in phase 2 and 3 clinical trials for the treatment of locally advanced and/or metastatic breast cancer with germline BRCA1/2 deleterious mutations.
Preclinical evaluation of the PARP inhibitor BMN-673 for the treatment of ovarian clear cell cancer.[Pubmed:28002809]
Oncotarget. 2017 Jan 24;8(4):6057-6066.
PURPOSE: To determine if models of ovarian clear cell carcinomas (OCCCs) harbouring defects in homologous recombination (HR) DNA repair of double strand breaks (DSBs) are sensitive to cisplatin and/or PARP inhibition. EXPERIMENTAL DESIGN: The HR status of 12 OCCC cell lines was determined using RAD51/gammaH2AX foci formation assays. Sensitivity to cisplatin and the PARP inhibitor BMN-673 was correlated with HR status. BRCA1, BRCA2, MRE11 and PTEN loss of expression was investigated as a potential determinant of BMN-673 sensitivity. A tissue microarray containing 50 consecutive primary OCCC was assessed for PTEN expression using immunohistochemistry. RESULTS: A subset of OCCC cells displayed reduced RAD51 foci formation in the presence of DNA DSBs, suggestive of HR defects. HR-defective OCCC cells, with the exception of KOC-7c, had higher sensitivity to cisplatin/ BMN-673 than HR-competent OCCC cell lines (Log10 SF50 -9.4 (SD +/- 0.29) vs -8.1 (SD +/- 0.35), mean difference 1.3, p < 0.01). Of the cell lines studied, two, TOV-21G and KOC-7c, showed loss of PTEN expression. In primary OCCCs, loss of PTEN expression was observed in 10% (5/49) of cases. CONCLUSIONS: A subset of OCCC cells are sensitive to PARP inhibition in vitro, which can be predicted by HR defects as defined by gammaH2AX/RAD51 foci formation. These results provide a rationale for the testing of HR deficiency and PARP inhibitors as a targeted therapy in a subset of OCCCs.


