Huperzine ACAS# 120786-18-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 120786-18-7 | SDF | Download SDF |
| PubChem ID | 5912039 | Appearance | Powder |
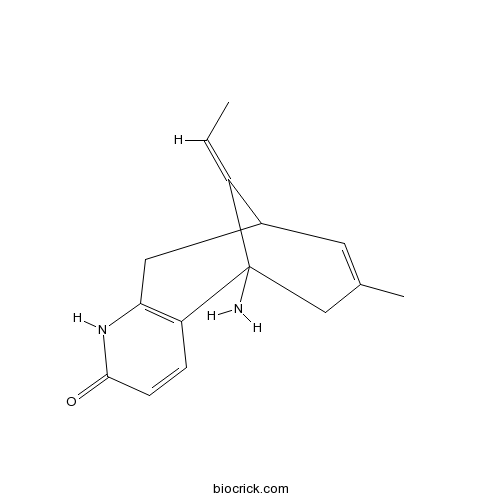
| Formula | C15H18N2O | M.Wt | 242.32 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Fordine; (+/-)-Huperzine A | ||
| Solubility | DMSO : ≥ 50 mg/mL (206.34 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (13E)-1-amino-13-ethylidene-11-methyl-6-azatricyclo[7.3.1.02,7]trideca-2(7),3,10-trien-5-one | ||
| SMILES | CC=C1C2CC3=C(C1(CC(=C2)C)N)C=CC(=O)N3 | ||
| Standard InChIKey | ZRJBHWIHUMBLCN-QDEBKDIKSA-N | ||
| Standard InChI | InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Huperzine A is a potent, selective and reversible acetylcholinesterase (AChE) inhibitor and has been widely used in China for the treatment of Alzheimer's disease (AD).Huperzine A induces CYP3A4 expression and activation via PXR dependent pathways, may contribute to drug-drug interactions with ligustrazine and oridonin. |
| Targets | P450 (e.g. CYP17) | AChR | Bcl-2/Bax | P53 | Caspase | PXR |
| In vitro | Huperzine A production by Paecilomyces tenuis YS-13, an endophytic fungus isolated from Huperzia serrata.[Pubmed: 25427833]Nat Prod Res. 2015 Jun;29(11):1035-41.Huperzine A (HupA), a naturally occurring alkaloid in the plant family Huperziaceae, has drawn great interest for its potential application in Alzheimer disease therapy. Our primary objective was to identify alkaloid- and HupA-producing fungi from the Chinese folk herb, Huperzia serrata. Huperzine A: Is it an Effective Disease-Modifying Drug for Alzheimer's Disease?[Pubmed: 25191267]Front Aging Neurosci. 2014 Aug 19;6:216.Alzheimer's disease (AD) is a progressive neurodegenerative disorder for which there is no cure. Huperzine A (HupA) is a natural inhibitor of acetylcholinesterase (AChE) derived from the Chinese folk medicine Huperzia serrata (Qian Ceng Ta). It is a licensed anti-AD drug in China and is available as a nutraceutical in the US.
Neuroprotective Effects of Huperzine A.[Reference: WebLink]Neurosignals, 2005, 14(1-2):71-82.Huperzine A (HupA), isolated from Chinese herb Huperzia serrata, is a potent, highly specific and reversible inhibitor of acetylcholinesterase. It has been found to reverse or attenuate cognitive deficits in a broad range of animal models. |
| Kinase Assay | Induction of human CYP3A4 by huperzine A, ligustrazine and oridonin through pregnane X receptor-mediated pathways.[Pubmed: 25073399 ]Pharmazie. 2014 Jul;69(7):532-6.The pregnane X receptor (PXR) is a key regulator of CYP3A4, which is involved in catalyzing the metabolic conversion of a number of endogenous substrates. |
| Structure Identification | PLoS One. 2015 Mar 23;10(3):e0120809.De Novo RNA Sequencing and Transcriptome Analysis of Colletotrichum gloeosporioides ES026 Reveal Genes Related to Biosynthesis of Huperzine A.[Pubmed: 25799531]Huperzine A is important in the treatment of Alzheimer's disease. There are major challenges for the mass production of Huperzine A from plants due to the limited number of Huperzine A-producing plants, as well as the low content of Huperzine A in these plants. Various endophytic fungi produce Huperzine A. Colletotrichum gloeosporioides ES026 was previously isolated from a Huperzine A-producing plant Huperzia serrata, and this fungus also produces Huperzine A. |

Huperzine A Dilution Calculator

Huperzine A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1268 mL | 20.6339 mL | 41.2677 mL | 82.5355 mL | 103.1694 mL |
| 5 mM | 0.8254 mL | 4.1268 mL | 8.2535 mL | 16.5071 mL | 20.6339 mL |
| 10 mM | 0.4127 mL | 2.0634 mL | 4.1268 mL | 8.2535 mL | 10.3169 mL |
| 50 mM | 0.0825 mL | 0.4127 mL | 0.8254 mL | 1.6507 mL | 2.0634 mL |
| 100 mM | 0.0413 mL | 0.2063 mL | 0.4127 mL | 0.8254 mL | 1.0317 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Huperzine A, an active Lycopodium alkaloid extracted from traditional Chinese herb, is a potent, selective and reversible acetylcholinesterase (AChE) inhibitor and has been widely used in China for the treatment of Alzheimer's disease (AD). IC50 value: Target: AChE Huperzine A exhibited protective effects against d-gal-induced hepatotoxicity and inflamm-aging by inhibiting AChE activity and via the activation of the cholinergic anti-inflammatory pathway. The huperzine A mechanism might be involved in the inhibition of DAMPs-mediated NF-κB nuclear localization and activation. Huperzine A is a potential therapeutic agent for Alzheimer's disease.
References:
[1]. Burshtein G, Friedman M, Greenberg S, Hoffman A. Transepithelial Transport of a Natural Cholinesterase Inhibitor, Huperzine A, along the Gastrointestinal Tract: the Role of Ionization on Absorption Mechanism. Planta Med. 2013 Jan 23.
[2]. Ruan Q, Liu F, Gao Z, et al. The anti-inflamm-aging and hepatoprotective effects of huperzine A in d-galactose-treated rats. Mech Ageing Dev. 2013 Jan 8. pii: S0047-6374(12)00182-0.
[3]. Zhang HY. New insights into huperzine A for the treatment of Alzheimer's disease. Acta Pharmacol Sin. 2012 Sep;33(9):1170-5.
[4]. Wang J, Zhang HY, Tang XC. Huperzine a improves chronic inflammation and cognitive decline in rats with cerebral hypoperfusion. J Neurosci Res. 2010 Mar;88(4):807-15. doi: 10.1002/jnr.22237.
[5]. Park P, Schachter S, Yaksh T. Intrathecal huperzine A increases thermal escape latency and decreases flinching behavior in the formalin test in rats. Neurosci Lett. 2010 Feb 5;470(1):6-9. doi: 10.1016/j.neulet.2009.12.033.
- 3,2'-Epilarixinol
Catalog No.:BCN6496
CAS No.:1207671-28-0
- LDV FITC
Catalog No.:BCC6229
CAS No.:1207610-07-8
- 5-OMe-UDP trisodium salt
Catalog No.:BCC6153
CAS No.:1207530-98-0
- BMN 673
Catalog No.:BCC2205
CAS No.:1207456-01-6
- BMN-673 8R,9S
Catalog No.:BCC1422
CAS No.:1207456-00-5
- GDC-0349
Catalog No.:BCC1094
CAS No.:1207360-89-1
- GDC-mTOR inhibitor
Catalog No.:BCC1781
CAS No.:1207358-59-5
- BI-847325
Catalog No.:BCC6511
CAS No.:1207293-36-4
- NVS-CRF38
Catalog No.:BCC8059
CAS No.:1207258-55-6
- 12alpha-Hydroxyevodol
Catalog No.:BCN6102
CAS No.:120722-04-5
- Sarcandrolide D
Catalog No.:BCN6621
CAS No.:1207185-03-2
- Scutebata G
Catalog No.:BCN6101
CAS No.:1207181-63-2
- Gynosaponin I
Catalog No.:BCN4078
CAS No.:1207861-69-5
- Quassidine B
Catalog No.:BCN7022
CAS No.:1207862-37-0
- CaMKII-IN-1
Catalog No.:BCC5530
CAS No.:1208123-85-6
- VU 0365114
Catalog No.:BCC6164
CAS No.:1208222-39-2
- Ketone Ester
Catalog No.:BCC1677
CAS No.:1208313-97-6
- N6022
Catalog No.:BCC4127
CAS No.:1208315-24-5
- PF-03394197(Oclacitinib)
Catalog No.:BCC6474
CAS No.:1208319-26-9
- Isoliquiritin apioside
Catalog No.:BCN2914
CAS No.:120926-46-7
- FPL 64176
Catalog No.:BCC7050
CAS No.:120934-96-5
- 3-Deazaneplanocin A (DZNep) hydrochloride
Catalog No.:BCC3604
CAS No.:120964-45-6
- Vanillin
Catalog No.:BCN2605
CAS No.:121-33-5
- Vanillic acid
Catalog No.:BCN6105
CAS No.:121-34-6
Huperzine A: Is it an Effective Disease-Modifying Drug for Alzheimer's Disease?[Pubmed:25191267]
Front Aging Neurosci. 2014 Aug 19;6:216.
Alzheimer's disease (AD) is a progressive neurodegenerative disorder for which there is no cure. Huperzine A (HupA) is a natural inhibitor of acetylcholinesterase (AChE) derived from the Chinese folk medicine Huperzia serrata (Qian Ceng Ta). It is a licensed anti-AD drug in China and is available as a nutraceutical in the US. A growing body of evidence has demonstrated that HupA has multifaceted pharmacological effects. In addition to the symptomatic, cognitive-enhancing effect via inhibition of AChE, a number of recent studies have reported that this drug has "non-cholinergic" effects on AD. Most important among these is the protective effect of HupA on neurons against amyloid beta-induced oxidative injury and mitochondrial dysfunction as well as via the up-regulation of nerve growth factor and antagonizing N-methyl-d-aspartate receptors. The most recent discovery that HupA may reduce brain iron accumulation lends further support to the argument that HupA could serve as a potential disease-modifying agent for AD and also other neurodegenerative disorders by significantly slowing down the course of neuronal death.
De novo RNA sequencing and transcriptome analysis of Colletotrichum gloeosporioides ES026 reveal genes related to biosynthesis of huperzine A.[Pubmed:25799531]
PLoS One. 2015 Mar 23;10(3):e0120809.
Huperzine A is important in the treatment of Alzheimer's disease. There are major challenges for the mass production of Huperzine A from plants due to the limited number of huperzine-A-producing plants, as well as the low content of Huperzine A in these plants. Various endophytic fungi produce Huperzine A. Colletotrichum gloeosporioides ES026 was previously isolated from a huperzine-A-producing plant Huperzia serrata, and this fungus also produces Huperzine A. In this study, de novo RNA sequencing of C. gloeosporioides ES026 was carried out with an Illumina HiSeq2000. A total of 4,324,299,051 bp from 50,442,617 high-quality sequence reads of ES026 were obtained. These raw data were assembled into 24,998 unigenes, 40,536,684 residues and 19,790 genes. The majority of the unique sequences were assigned to corresponding putative functions based on BLAST searches of public databases. The molecular functions, biological processes and biochemical pathways of these unique sequences were determined using gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) assignments. A gene encoding copper amine oxidase (CAO) (unigene 9322) was annotated for the conversion of cadaverine to 5-aminopentanal in the biosynthesis of Huperzine A. This gene was also detected in the root, stem and leaf of H. serrata. Furthermore, a close relationship was observed between expression of the CAO gene (unigene 9322) and quantity of crude Huperzine A extracted from ES026. Therefore, CAO might be involved in the biosynthesis of Huperzine A and it most likely plays a key role in regulating the content of Huperzine A in ES026.
Huperzine A production by Paecilomyces tenuis YS-13, an endophytic fungus isolated from Huperzia serrata.[Pubmed:25427833]
Nat Prod Res. 2015;29(11):1035-41.
Huperzine A (HupA), a naturally occurring alkaloid in the plant family Huperziaceae, has drawn great interest for its potential application in Alzheimer disease therapy. Our primary objective was to identify alkaloid- and HupA-producing fungi from the Chinese folk herb, Huperzia serrata. We established a rapid and efficient model for screening HupA-producing endophytic fungal strains. The presence of HupA in Paecilomyces tenuis YS-13 was analysed by thin-layer chromatography, high-performance liquid chromatography and mass spectrometry. The fermentation yield of HupA was 21.0 mug/L, and the IC50 of the crude extract of YS-13 fermentation broth was 1.27 +/- 0.04 mg/mL. This is the first report of P. tenuis as a HupA-producing endophyte isolated from Huperziaceae.
Induction of human CYP3A4 by huperzine A, ligustrazine and oridonin through pregnane X receptor-mediated pathways.[Pubmed:25073399]
Pharmazie. 2014 Jul;69(7):532-6.
The pregnane X receptor (PXR) is a key regulator of CYP3A4, which is involved in catalyzing the metabolic conversion of a number of endogenous substrates. In this study, we screened 22 compounds isolated from traditional Chinese herbal medicines using luciferase reporter gene assays for inspecting their capabilities in inducing PXR-mediated transactivation of CYP3A4 expression. In addition, the mRNA and protein expressions of CYP3A4 and PXR as well as the enzymatic activites of CYP3A4 were analyzed by real-time PCR, Western blot analysis and UPLC-MS/MS-based metabolite assay in LS174T cells. Huperzine A, ligustrazine and oridonin were identified to be the inducers of CYP3A4. These compounds induced the CYP3A4 reporter luciferase activity, and up-regulated CYP3A4 mRNA and protein levels significantly. Besides, Huperzine A, ligustrazine and oridonin significantly up-regulated enzymatic activities of CYP3A4. However, the three compounds showed no effects on PXR mRNA and protein expression. To our knowledge, it is the first identification of these three compounds as PXR activators to induce CYP3A4. These results indicate that Huperzine A, ligustrazine and oridonin induced CYP3A4 expression and activation via PXR dependent pathways, and might contribute to drug-drug interactions.


