LY2606368CHK1 inhibitor CAS# 1234015-52-1 |
- LY2606368
Catalog No.:BCC4105
CAS No.:1234015-52-1
- CHIR-124
Catalog No.:BCC3750
CAS No.:405168-58-3
- BML-277
Catalog No.:BCC4245
CAS No.:516480-79-8
- AZD7762
Catalog No.:BCC2555
CAS No.:860352-01-8
- MK-8776 (SCH-900776)
Catalog No.:BCC3817
CAS No.:891494-63-6
- PF-477736
Catalog No.:BCC4421
CAS No.:952021-60-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1234015-52-1 | SDF | Download SDF |
| PubChem ID | 46700756 | Appearance | Powder |
| Formula | C18H19N7O2 | M.Wt | 365.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Prexasertib | ||
| Solubility | DMSO : 16.67 mg/mL (45.62 mM; Need ultrasonic) | ||
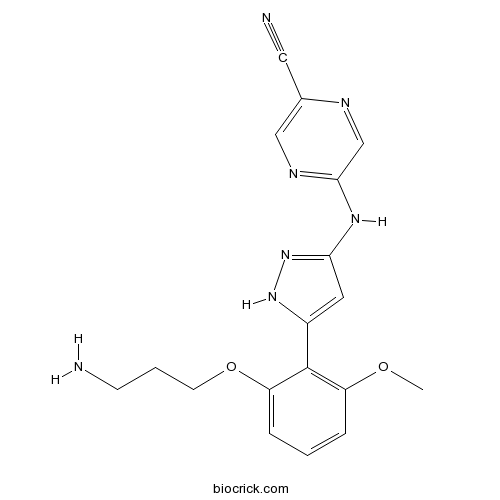
| Chemical Name | 5-[[5-[2-(3-aminopropoxy)-6-methoxyphenyl]-1H-pyrazol-3-yl]amino]pyrazine-2-carbonitrile | ||
| SMILES | COC1=C(C(=CC=C1)OCCCN)C2=CC(=NN2)NC3=NC=C(N=C3)C#N | ||
| Standard InChIKey | DOTGPNHGTYJDEP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H19N7O2/c1-26-14-4-2-5-15(27-7-3-6-19)18(14)13-8-16(25-24-13)23-17-11-21-12(9-20)10-22-17/h2,4-5,8,10-11H,3,6-7,19H2,1H3,(H2,22,23,24,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | LY2606368 is a potent and selective ATP competitive inhibitor of the Chk1 protein kinase, with IC50s of <1 nM and 8 nM for CHK1 and CHK2, respectively, and a Ki of 0.9 nM against purified CHK1.In Vitro:LY2606368 is a potent and selective ATP competitive inhibitor of Chk1, with an IC50 of <1 nM, and also inhibits CHK2, with an IC50 of 8 nM. LY2606368 has an EC50 of 1 nM for CHK1 activity through autophosphorylation of serine 296 and <31 nM for HT-29 CHK2 autophosphorylation (S516). LY2606368 potently abrogates the G2-M checkpoint activated by doxorubicin in p53-deficient HeLa cells with an EC50 of 9 nM. However, 100 nM LY2606368 does not inhibit PMA-stimulated RSK but instead weakly stimulates phosphorylation of S6 on serines 235/236. LY2606368 is broadly antiproliferative with IC50s of 3 nM, 3 nM, 10 nM, 37 nM, and 68 nM against U-2 OS, Calu-6, HT-29, HeLa, and NCI-H460 cell lines, respectively. LY2606368 (4 nM) results in a large shift in cell-cycle populations from G1 and G2-M to S-phase with an accompanied induction of H2AX phosphorylation in U-2 OS cells[1]. LY2606368 (25 μM) exhibits inhibitory activities against proliferation of AGS and MKN1 cells. LY2606368 (20 nM) inhibits HR repair capacity DR-GFP cells. LY2606368 (5 nM) in combination with PARP inhibitor BMN673, displays synergistic anticancer effects in gastric cancer cells[2].In Vivo:LY2606368 (15 mg/kg, s.c.) significantly inhibits tumor growth in xenograft tumor models with less animal weight loss[1]. LY2606368 (2 mg/kg, s.c.) and BMN673 combination has synergistic anticancer effect in gastric cancer PDX model, and the effect is higher than that of one drug alone[2]. References: | |||||
| Cell experiment [1]: | |
| Cell lines | Hela cells |
| Preparation method | Limited solubility. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
| Reacting condition | 7 h |
| Applications | LY2606368 triggers DNA damage during S-phase as pH2AX (S139) and TUNEL-positive staining cells increases substantially in Sphase cells. LY2606368 also need CDC25A and CDK2 to trigger DNA damage. In addition, LY2606368 leads to replication catastrophe. |
| Animal experiment [1]: | |
| Animal models | Female CD-1 nu-/nu- mice (26–28 g) bearing Calu-6 tumor |
| Dosage form | Twice daily for 3 days with 1, 3.3, or 10 mg/kg of LY2606368 |
| Application | Up to 72.3% tumor growth inhibition is observed in all three doses of LY2606368 groups. Wight loss of mice is not exceeded by 3%, indicating the LY2606368 is well tolerated in any of the treatment groups. Moreover, tumor regrowth of the highest dose group is slow during the 28-day recovery period, suggesting a durable response to LY2606368. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: 1. King C, Diaz HB, McNeely S et al. LY2606368 Causes Replication Catastrophe and Antitumor Effects through CHK1-Dependent Mechanisms. Mol Cancer Ther. 2015 Sep;14(9):2004-13. | |

LY2606368 Dilution Calculator

LY2606368 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7368 mL | 13.684 mL | 27.368 mL | 54.736 mL | 68.42 mL |
| 5 mM | 0.5474 mL | 2.7368 mL | 5.4736 mL | 10.9472 mL | 13.684 mL |
| 10 mM | 0.2737 mL | 1.3684 mL | 2.7368 mL | 5.4736 mL | 6.842 mL |
| 50 mM | 0.0547 mL | 0.2737 mL | 0.5474 mL | 1.0947 mL | 1.3684 mL |
| 100 mM | 0.0274 mL | 0.1368 mL | 0.2737 mL | 0.5474 mL | 0.6842 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LY2606368 is a selective ATP competitive inhibitor of checkpoint kinase 1(CHK1) with IC50 value of 1.5nM in SW1990 cells [1].
CHK1 is an intracellular serine/threonine kinase that plays a role in DNA damage response pathway. The inhibitors of CHK1 are developed for the treatment of cancers. LY2606368 is an ATP-competitive inhibitor of CHK1 and is undergoing clinical trials currently. It inhibits the auto-phosphorylation of CHK1 and induces the phosphorylation of H2AX in cancer cells. In the pancreatic cell line SW1990, LY2606368 significantly inhibits cell proliferation with IC50 value of 1.5nM. LY2606368 also exerts potent anti-tumor activity in SW1990 xenograft model. Besides that, in the orthotopic SKVO3 model, treatment of LY2606368 is found to inhibit tumor growth and reduce the incidence of metastases and accumulation. However, LY2606368 is only administered intravenously due to its poor oral bioavailability [1, 2 and 3].
References:
[1] Wu W, Bi C, Bence A K, et al. Antitumor activity of Chk1 inhibitor LY2606368 as a single agent in SW1990 human pancreas orthotopic tumor model. Cancer Research, 2012, 72(8 Supplement): 1776.
[2] Lainchbury M, Matthews T P, McHardy T, et al. Discovery of 3-alkoxyamino-5-(pyridin-2-ylamino) pyrazine-2-carbonitriles as selective, orally bioavailable CHK1 inhibitors. Journal of medicinal chemistry, 2012, 55(22): 10229-10240.
[3] McNeely S C, Burke T F, DurlandBusbice S, et al. Abstract A108: LY2606368, a second generation Chk1 inhibitor, inhibits growth of ovarian carcinoma xenografts either as monotherapy or in combination with standard-of-care agents. Molecular Cancer Therapeutics, 2011, 10(Supplement 1): A108.
- Z-Arg-OH
Catalog No.:BCC3060
CAS No.:1234-35-1
- ELR510444
Catalog No.:BCC6418
CAS No.:1233948-35-0
- Methyl 4-caffeoylquinate
Catalog No.:BCN3442
CAS No.:123372-74-7
- CAY10650
Catalog No.:BCC4178
CAS No.:1233706-88-1
- kb NB 142-70
Catalog No.:BCC1675
CAS No.:1233533-04-4
- Dipsacobioside
Catalog No.:BCN6552
CAS No.:123350-57-2
- AZ20
Catalog No.:BCC1389
CAS No.:1233339-22-4
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- 2'-O-Methylhelichrysetin
Catalog No.:BCN4792
CAS No.:123316-64-3
- 6alpha-Hydroxytomentosin
Catalog No.:BCN7303
CAS No.:1232676-22-0
- VE-821
Catalog No.:BCC1207
CAS No.:1232410-49-9
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- Boc-Glu(Ofm)-OH
Catalog No.:BCC3391
CAS No.:123417-18-5
- Rivastigmine
Catalog No.:BCC1900
CAS No.:123441-03-2
- XMD8-92
Catalog No.:BCC2062
CAS No.:1234480-50-2
- LRRK2-IN-1
Catalog No.:BCC1706
CAS No.:1234480-84-2
- PM 102
Catalog No.:BCC6105
CAS No.:1234564-95-4
- PNU 282987
Catalog No.:BCC7318
CAS No.:123464-89-1
- LY2608204
Catalog No.:BCC4969
CAS No.:1234703-40-2
- SAR191801
Catalog No.:BCC6393
CAS No.:1234708-04-3
- 3-O-Caffeoylquinic acid methyl ester
Catalog No.:BCN3484
CAS No.:123483-19-2
- Demethylmurrayanine
Catalog No.:BCN4721
CAS No.:123497-84-7
- Biperiden HCl
Catalog No.:BCC4565
CAS No.:1235-82-1
- Linderaspirone A
Catalog No.:BCN6122
CAS No.:1235126-46-1
Phase I Study of LY2606368, a Checkpoint Kinase 1 Inhibitor, in Patients With Advanced Cancer.[Pubmed:27044938]
J Clin Oncol. 2016 May 20;34(15):1764-71.
PURPOSE: The primary objective was to determine safety, toxicity, and a recommended phase II dose regimen of LY2606368, an inhibitor of checkpoint kinase 1, as monotherapy. PATIENTS AND METHODS: This phase I, nonrandomized, open-label, dose-escalation trial used a 3 + 3 dose-escalation scheme and included patients with advanced solid tumors. Intravenous LY2606368 was dose escalated from 10 to 50 mg/m(2) on schedule 1 (days 1 to 3 every 14 days) or from 40 to 130 mg/m(2) on schedule 2 (day 1 every 14 days). Safety measures and pharmacokinetics were assessed, and pharmacodynamics were measured in blood, hair follicles, and circulating tumor cells. RESULTS: Forty-five patients were treated; seven experienced dose-limiting toxicities (all hematologic). The maximum-tolerated doses (MTDs) were 40 mg/m(2) (schedule 1) and 105 mg/m(2) (schedule 2). The most common related grade 3 or 4 treatment-emergent adverse events were neutropenia, leukopenia, anemia, thrombocytopenia, and fatigue. Grade 4 neutropenia occurred in 73.3% of patients and was transient (typically < 5 days). Febrile neutropenia incidence was low (7%). The LY2606368 exposure over the first 72 hours (area under the curve from 0 to 72 hours) at the MTD for each schedule coincided with the exposure in mouse xenografts that resulted in maximal tumor responses. Minor intra- and intercycle accumulation of LY2606368 was observed at the MTDs for both schedules. Two patients (4.4%) had a partial response; one had squamous cell carcinoma (SCC) of the anus and one had SCC of the head and neck. Fifteen patients (33.3%) had a best overall response of stable disease (range, 1.2 to 6.7 months), six of whom had SCC. CONCLUSION: An LY2606368 dose of 105 mg/m(2) once every 14 days is being evaluated as the recommended phase II dose in dose-expansion cohorts for patients with SCC.
LY2606368 Causes Replication Catastrophe and Antitumor Effects through CHK1-Dependent Mechanisms.[Pubmed:26141948]
Mol Cancer Ther. 2015 Sep;14(9):2004-13.
CHK1 is a multifunctional protein kinase integral to both the cellular response to DNA damage and control of the number of active replication forks. CHK1 inhibitors are currently under investigation as chemopotentiating agents due to CHK1's role in establishing DNA damage checkpoints in the cell cycle. Here, we describe the characterization of a novel CHK1 inhibitor, LY2606368, which as a single agent causes double-stranded DNA breakage while simultaneously removing the protection of the DNA damage checkpoints. The action of LY2606368 is dependent upon inhibition of CHK1 and the corresponding increase in CDC25A activation of CDK2, which increases the number of replication forks while reducing their stability. Treatment of cells with LY2606368 results in the rapid appearance of TUNEL and pH2AX-positive double-stranded DNA breaks in the S-phase cell population. Loss of the CHK1-dependent DNA damage checkpoints permits cells with damaged DNA to proceed into early mitosis and die. The majority of treated mitotic nuclei consist of extensively fragmented chromosomes. Inhibition of apoptosis by the caspase inhibitor Z-VAD-FMK had no effect on chromosome fragmentation, indicating that LY2606368 causes replication catastrophe. Changes in the ratio of RPA2 to phosphorylated H2AX following LY2606368 treatment further support replication catastrophe as the mechanism of DNA damage. LY2606368 shows similar activity in xenograft tumor models, which results in significant tumor growth inhibition. LY2606368 is a potent representative of a novel class of drugs for the treatment of cancer that acts through replication catastrophe.


