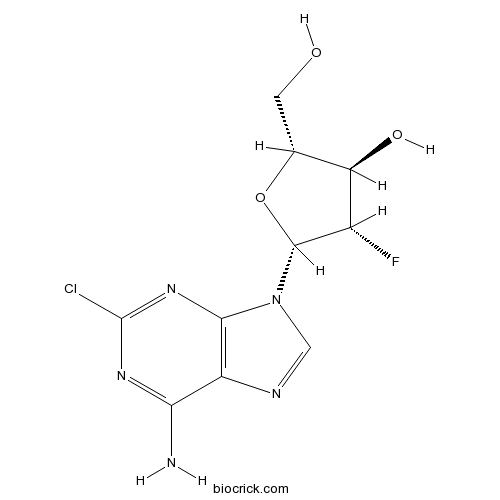
ClofarabineAntimetabolite,inhibit DNA polymerase and ribonucleotide reductase CAS# 123318-82-1 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
- Carboplatin
Catalog No.:BCC1170
CAS No.:41575-94-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 123318-82-1 | SDF | Download SDF |
| PubChem ID | 119182 | Appearance | Powder |
| Formula | C10H11ClFN5O3 | M.Wt | 303.68 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Clolar; Evoltra; Clofarex; CAFdA | ||
| Solubility | DMSO : ≥ 50 mg/mL (164.65 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (2R,3R,4S,5R)-5-(6-amino-2-chloropurin-9-yl)-4-fluoro-2-(hydroxymethyl)oxolan-3-ol | ||
| SMILES | C1=NC2=C(N1C3C(C(C(O3)CO)O)F)N=C(N=C2N)Cl | ||
| Standard InChIKey | WDDPHFBMKLOVOX-AYQXTPAHSA-N | ||
| Standard InChI | InChI=1S/C10H11ClFN5O3/c11-10-15-7(13)5-8(16-10)17(2-14-5)9-4(12)6(19)3(1-18)20-9/h2-4,6,9,18-19H,1H2,(H2,13,15,16)/t3-,4+,6-,9-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Deoxycytidine kinase (dCK) substrate. Phosphorylated to form clofarabine triphosphate, which competes with dATP for DNA polymerase-α and -ε and potently inhibits ribonucleotide reductase (IC50 = 65 nM). Induces apoptosis by directly altering mitochondrial transmembrane potential. Demonstrates growth inhibition and cytotoxic activity in a variety of leukemias and solid tumors. |

Clofarabine Dilution Calculator

Clofarabine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2929 mL | 16.4647 mL | 32.9294 mL | 65.8588 mL | 82.3235 mL |
| 5 mM | 0.6586 mL | 3.2929 mL | 6.5859 mL | 13.1718 mL | 16.4647 mL |
| 10 mM | 0.3293 mL | 1.6465 mL | 3.2929 mL | 6.5859 mL | 8.2323 mL |
| 50 mM | 0.0659 mL | 0.3293 mL | 0.6586 mL | 1.3172 mL | 1.6465 mL |
| 100 mM | 0.0329 mL | 0.1646 mL | 0.3293 mL | 0.6586 mL | 0.8232 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Clofarabine is a DNA synthesis inhibitor and a substrate of Deoxycytidine kinase (dCK).
DCK is a key cytosolic enzyme in the DNA-synthesis salvage pathway and is responsible for the phosphorylation of clofarabine.
Clofarabine is phosphorylated to form clofarabine triphosphate, which competes with dATP for DNA polymerase-α and -ε. At the same time, clofarabine-monophosphate is incorporated into internal and terminal DNA sites, which impaired DNA elongation and repair. Clofarabine triphosphate inhibits ribonucleotide reductase with IC50 value of 65 nM, which then reduced dCTP and dATP. Clofarabine is efficiently transported into cells through nucleoside transporters hENT1, hENT2, and hCNT2. Clofarabine results in release of cytochrome c, apoptosis protease-activating factor 1 (APAF1), apoptotic-inducing factor (AIF) and caspase 9 into the cytosol. In both rapidly growing and quiescent tumours, clofarabine has anticancer activity because of its inhibition of DNA synthesis and induction of apoptosis.
Implanted human tumour xenografts in athymic nude or severe combined immune deficiency mice, Clofarabine administered intraperitoneally had significant antitumor activity.
References:
[1]. Bonate PL, Arthaud L, Cantrell WR Jr, et al. Discovery and development of clofarabine: a nucleoside analogue for treating cancer. Nat Rev Drug Discov, 2006, 5(10): 855-863.
- 2'-O-Methylhelichrysetin
Catalog No.:BCN4792
CAS No.:123316-64-3
- 6alpha-Hydroxytomentosin
Catalog No.:BCN7303
CAS No.:1232676-22-0
- VE-821
Catalog No.:BCC1207
CAS No.:1232410-49-9
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- LY2835219 free base
Catalog No.:BCC1722
CAS No.:1231929-97-7
- Cefepime Dihydrochloride Monohydrate
Catalog No.:BCC5261
CAS No.:123171-59-5
- Uncaric acid
Catalog No.:BCN6121
CAS No.:123135-05-7
- 4,8-Dihydroxyeudesm-7(11)-en-12,8-olide
Catalog No.:BCN1600
CAS No.:1231208-53-9
- BAF312 (Siponimod)
Catalog No.:BCC5114
CAS No.:1230487-00-9
- Phyltetralin
Catalog No.:BCN3051
CAS No.:123048-17-9
- Bulleyanin
Catalog No.:BCN6120
CAS No.:123043-54-9
- Azasetron HCl
Catalog No.:BCC5035
CAS No.:123040-16-4
- AZ20
Catalog No.:BCC1389
CAS No.:1233339-22-4
- Dipsacobioside
Catalog No.:BCN6552
CAS No.:123350-57-2
- kb NB 142-70
Catalog No.:BCC1675
CAS No.:1233533-04-4
- CAY10650
Catalog No.:BCC4178
CAS No.:1233706-88-1
- Methyl 4-caffeoylquinate
Catalog No.:BCN3442
CAS No.:123372-74-7
- ELR510444
Catalog No.:BCC6418
CAS No.:1233948-35-0
- Z-Arg-OH
Catalog No.:BCC3060
CAS No.:1234-35-1
- LY2606368
Catalog No.:BCC4105
CAS No.:1234015-52-1
- Boc-Glu(Ofm)-OH
Catalog No.:BCC3391
CAS No.:123417-18-5
- Rivastigmine
Catalog No.:BCC1900
CAS No.:123441-03-2
- XMD8-92
Catalog No.:BCC2062
CAS No.:1234480-50-2
- LRRK2-IN-1
Catalog No.:BCC1706
CAS No.:1234480-84-2
Clofarabine desensitization: A case report: Leukemia research reports.[Pubmed:28229039]
Leuk Res Rep. 2017 Feb 13;7:14-16.
We describe a relapsed AML patient who had two prior severe reactions to Clofarabine involving rigors, emesis, tachycardia, hypotension, and acute kidney injury. Given previous prolonged remission achieved with Clofarabine and cytarabine therapy years prior, rechallenge was undertaken upon discovery of AML relapse. We designed a desensitization protocol performed with the first dose of Clofarabine, leading to successful administration of the entire Clofarabine/cytarabine treatment course. From this case we show promise for Clofarabine rechallenge after prior hypersensitivity reactions in patients with few treatment options for relapsed AML.
Clofarabine-based chemotherapy as a bridge to transplant in the setting of refractory or relapsed acute myeloid leukemia, after at least one previous unsuccessful salvage treatment containing fludarabine: a single institution experience.[Pubmed:28220349]
Int J Hematol. 2017 Jun;105(6):769-776.
For refractory or relapsed acute myeloid leukemia patients, allogeneic hematopoietic stem cell transplantation is the only curative treatment option, but the disease must be in remission before this can be attempted. "Salvage" therapy regimens containing high-dose cytarabine plus fludarabine or cladribine with or without anthracyclines or plus mitoxantrone and etoposide fail in 30-50% of cases. We report the outcome of 14 patients treated with a Clofarabine-based treatment administered after at least one failed fludarabine-based "salvage" attempt in a "real life" (outside a clinical trial) context. No death related to the Clofarabine-based treatment was observed. Four of the 14 patients (29%) reached complete remission and one (7%) achieved a reduction of marrow blasts to fewer than 10%. Three of these five patients were successfully transplanted and have shown a long-term survival. The small number of this group of patients does not permit the identification of clinical features clearly related to a favorable outcome, but we note that all the three long-term survivals were FLT3 wild type. Clofarabine-based "salvage therapy" in patients with very poor expectancy is feasible even after a fludarabine-based salvage attempt, albeit with success only in a small percentage of cases (3/14 = 21%).
Randomized Phase II Study of Clofarabine-Based Consolidation for Younger Adults With Acute Myeloid Leukemia in First Remission.[Pubmed:28221862]
J Clin Oncol. 2017 Apr 10;35(11):1223-1230.
Purpose To evaluate the efficacy and safety of a Clofarabine-based combination (CLARA) versus conventional high-dose cytarabine (HDAC) as postremission chemotherapy in younger patients with acute myeloid leukemia (AML). Patients and Methods Patients age 18 to 59 years old with intermediate- or unfavorable-risk AML in first remission and no identified donor for allogeneic stem-cell transplantation (SCT) were eligible. Two hundred twenty-one patients were randomly assigned to receive three CLARA or three HDAC consolidation cycles. The primary end point was relapse-free survival (RFS). To handle the confounding effect of SCT that could occur in patients with late donor identification, hazard ratios (HRs) of events were adjusted on the time-dependent treatment x SCT interaction term. Results At 2 years, RFS was 58.5% (95% CI, 49% to 67%) in the CLARA arm and 46.5% (95% CI, 37% to 55%) in the HDAC arm. Overall, 110 patients (55 in each arm) received SCT in first remission. On the basis of a multivariable Cox-adjusted treatment x SCT interaction, the HR of CLARA over HDAC before or in absence of SCT was 0.65 (95% CI, 0.43 to 0.98; P = .041). In a sensitivity analysis, when patients who received SCT in first remission were censored at SCT time, 2-year RFS was 53.3% (95% CI, 39% to 66%) in the CLARA arm and 31.0% (95% CI, 19% to 43%) in the HDAC arm (HR, 0.63; 95% CI, 0.41 to 0.98; P = .043). Gain in RFS could be related to the lower cumulative incidence of relapse observed in the CLARA arm versus the HDAC arm (33.9% v 46.4% at 2 years, respectively; cause-specific HR, 0.61; 95% CI, 0.40 to 0.94; P = .025). CLARA cycles were associated with higher hematologic and nonhematologic toxicity than HDAC cycles. Conclusion These results suggest that CLARA might be considered as a new chemotherapy option in younger patients with AML in first remission.
Discovery and development of clofarabine: a nucleoside analogue for treating cancer.[Pubmed:17016426]
Nat Rev Drug Discov. 2006 Oct;5(10):855-63.
The treatment of acute leukaemias, which are the most common paediatric cancers, has improved considerably in recent decades, with complete response rates approaching approximately 90% in some cases. However, there remains a major need for treatments for patients who do not achieve or maintain complete remission, for whom the prognosis is very poor. In this article, we describe the challenges involved in the discovery and development of Clofarabine, a second-generation nucleoside analogue that received accelerated approval from the US FDA at the end of 2004 for the treatment of paediatric patients 1-21 years old with relapsed or refractory acute lymphoblastic leukaemia after at least two prior regimens. It is the first such drug to be approved for paediatric leukaemia in more than a decade, and the first to receive approval for paediatric use before adult use.
Effects of 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)adenine on K562 cellular metabolism and the inhibition of human ribonucleotide reductase and DNA polymerases by its 5'-triphosphate.[Pubmed:1707752]
Cancer Res. 1991 May 1;51(9):2386-94.
2-Chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)-adenine (Cl-F-ara-A) has activity against the P388 tumor in mice on several different schedules. Biochemical studies with a chronic myelogenous leukemia cell line (K562) grown in cell culture have been done in order to better understand its mechanism of action. Cl-F-ara-A was a potent inhibitor of K562 cell growth. Only 5 nM inhibited K562 cell growth by 50% after 72 h of continuous incubation. The 5'-triphosphate of Cl-F-ara-A was detected by strong anion exchange chromatography of the acid-soluble extract of K562 cells incubated with Cl-F-ara-A. Competition studies with natural nucleosides suggested that deoxycytidine kinase was the enzyme responsible for the metabolism to the monophosphate. Incubation of K562 cells for 4 h with 50 nM Cl-F-ara-A inhibited the incorporation of [3H]thymidine into the DNA by 50%. Incubation with 0.1, 1, or 10 microM Cl-F-ara-A for 4 h depressed dATP, dCTP, and dGTP pools but did not affect TTP pools. Similar inhibition of deoxyribonucleoside triphosphate pools was seen after incubation with 2-chloro-2'-deoxyadenosine. Both Cl-F-ara-ATP and Cl-dATP potently inhibited the reduction of ADP to dADP in crude extracts of K562 cells (concentration producing 50% inhibition, 65 nM). The effect of Cl-F-ara-ATP on human DNA polymerases alpha, beta, and gamma isolated from K562 cells grown in culture was determined and compared with those of Cl-dATP and 9-beta-D-arabinofuranosyl-2-fluoroadenine triphosphate (F-ara-ATP). Cl-F-ara-ATP was a potent inhibitor of DNA polymerase alpha. Inhibition of DNA polymerase alpha was competitive with respect to dATP (Ki of 1 microM). The three analogue triphosphates were incorporated into the DNA by DNA polymerase alpha as efficiently as dATP. The incorporation of Cl-F-ara-AMP inhibited the further elongation of the DNA chain, similarly to that seen after the incorporation of F-ara-AMP. Extension of the DNA chain after the incorporation of Cl-dAMP was not inhibited as much as it was with either Cl-F-ara-AMP or F-ara-AMP. Cl-F-ara-ATP was not a potent inhibitor of DNA polymerase beta, DNA polymerase gamma, or DNA primase.(ABSTRACT TRUNCATED AT 400 WORDS)


