CarboplatinAntitumor agent that forms platinum-DNA adducts. CAS# 41575-94-4 |
- GAP-134
Catalog No.:BCC1588
CAS No.:943134-39-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 41575-94-4 | SDF | Download SDF |
| PubChem ID | 5352133 | Appearance | Powder |
| Formula | C6H12N2O4Pt | M.Wt | 371.25 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NSC 241240, Paraplatin, JM 8 | ||
| Solubility | H2O : 4.9 mg/mL (13.20 mM; Need ultrasonic and warming; DMSO can inactivate Carboplatin's activity) DMF : < 1 mg/mL (insoluble; DMSO can inactivate Carboplatin's activity) | ||
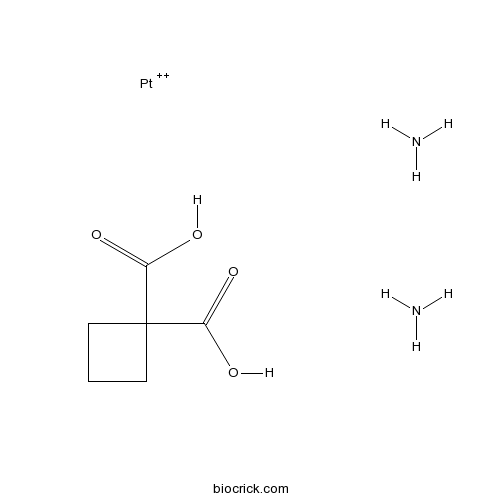
| Chemical Name | azane;cyclobutane-1,1-dicarboxylic acid;platinum(2+) | ||
| SMILES | C1CC(C1)(C(=O)O)C(=O)O.N.N.[Pt+2] | ||
| Standard InChIKey | OLESAACUTLOWQZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H8O4.2H3N.Pt/c7-4(8)6(5(9)10)2-1-3-6;;;/h1-3H2,(H,7,8)(H,9,10);2*1H3;/q;;;+2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antitumor agent that forms platinum-DNA adducts. Causes intra- and interstrand DNA crosslinks blocking DNA replication and transcription. Enhances radiation-induced single-strand DNA breakage and displays lower nephrotoxicity than analog cisplatin. |

Carboplatin Dilution Calculator

Carboplatin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6936 mL | 13.468 mL | 26.936 mL | 53.8721 mL | 67.3401 mL |
| 5 mM | 0.5387 mL | 2.6936 mL | 5.3872 mL | 10.7744 mL | 13.468 mL |
| 10 mM | 0.2694 mL | 1.3468 mL | 2.6936 mL | 5.3872 mL | 6.734 mL |
| 50 mM | 0.0539 mL | 0.2694 mL | 0.5387 mL | 1.0774 mL | 1.3468 mL |
| 100 mM | 0.0269 mL | 0.1347 mL | 0.2694 mL | 0.5387 mL | 0.6734 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Carboplatin is a DNA synthesis inhibitor.
DNA synthesis is the natural or artificial creation of deoxyribonucleic acid (DNA) molecules.
Carboplatin inhibits DNA synthesis by binding to DNA and interfering with repair mechanism. In MTT assays with A2780, SKOV-3, IGROV-1 and HX62 human ovarian cancer cells, Carboplatin inhibited cell proliferation with IC50 of 6.2 μM, 12.4 μM, 2.2 μM and 116 μM for A2780, SKOV-3, IGROV-1 and HX62, respectively [1]. In UMC-11, H727 and H835 lung carcinoid cell line, Carboplatin also shows the anti-proliferative activities [2].
In xenograft-bearing mice, Carboplatin (60 mg/kg) had a modest antitumor effect and the relative tumor volumes on day 6 were 8.4 relative to the control of 11.9 [1]. Treatment of 56 small cell lung carcinoma patients with Carboplatin (300-400 mg/m2), 23 patients achieved a response including 5 complete remissions. 18 of 30 previously untreated patients achieved a response. Carboplatin was well tolerated and there was no nephrotoxicity [3].
References:
[1]. Banerji U, Sain N, Sharp SY, et al. An in vitro and in vivo study of the combination of the heat shock protein inhibitor 17-allylamino-17-demethoxygeldanamycin and carboplatin in human ovarian cancer models. Cancer Chemother Pharmacol, 2008, 62(5): 769-778.
[2]. Fiebiger W, Olszewski U, Ulsperger E, et al. In vitro cytotoxicity of novel platinum-based drugs and dichloroacetate against lung carcinoid cell lines. Clin Transl Oncol, 2011, 13(1): 43-49.
[3]. Smith IE, Evans BD. Carboplatin (JM8) as a single agent and in combination in the treatment of small cell lung cancer. Cancer Treat Rev, 1985, 12 Suppl A: 73-75.
- Poricoic acid G
Catalog No.:BCN8267
CAS No.:415724-84-4
- RI-1
Catalog No.:BCC1896
CAS No.:415713-60-9
- SN-6
Catalog No.:BCC7273
CAS No.:415697-08-4
- N6-Cyclopentyladenosine
Catalog No.:BCC7160
CAS No.:41552-82-3
- 1-(3,4-dimethoxyphenyl)-2-(4-allly-2,6-dimethoxyphenoxy)propan-1-ol
Catalog No.:BCN1445
CAS No.:41535-95-9
- 2,6,16-Kauranetriol
Catalog No.:BCN5472
CAS No.:41530-90-9
- Koaburaside monomethyl ether
Catalog No.:BCN5471
CAS No.:41514-64-1
- Daturaolone
Catalog No.:BCN3904
CAS No.:41498-80-0
- 12-Oleanene-3,6-diol
Catalog No.:BCN3903
CAS No.:41498-79-7
- 3-Amino-1-phenyl-2-pyrazolin-5-one
Catalog No.:BCC8605
CAS No.:4149-06-8
- Belinostat (PXD101)
Catalog No.:BCC2153
CAS No.:414864-00-9
- Turkesterone
Catalog No.:BCN2363
CAS No.:41451-87-0
- 4,R-ajmalicine N-oxide
Catalog No.:BCN5473
CAS No.:41590-29-8
- (-)-Pinoresinol 4-O-glucoside
Catalog No.:BCN7251
CAS No.:41607-20-9
- Ylangenol
Catalog No.:BCN6705
CAS No.:41610-69-9
- Ciclopirox ethanolamine
Catalog No.:BCC4372
CAS No.:41621-49-2
- Lacinilene C
Catalog No.:BCN5474
CAS No.:41653-72-9
- Koaburaside
Catalog No.:BCN5475
CAS No.:41653-73-0
- 7-O-Methyl morroniside
Catalog No.:BCN3882
CAS No.:41679-97-4
- Catharanthine hemitartrate
Catalog No.:BCN8463
CAS No.:4168-17-6
- 2H-1-Benzopyran-7-yloxy
Catalog No.:BCN3580
CAS No.:41680-08-4
- 4',7-Dihydroxyflavanone
Catalog No.:BCC8333
CAS No.:41680-09-5
- 8-Methyleugenitol
Catalog No.:BCN6459
CAS No.:41682-21-7
- Arctigenin 4'-O-beta-gentiobioside
Catalog No.:BCN2847
CAS No.:41682-24-0
Efficacy of the PARP Inhibitor Veliparib with Carboplatin or as a Single Agent in Patients with Germline BRCA1- or BRCA2-Associated Metastatic Breast Cancer: California Cancer Consortium Trial NCT01149083.[Pubmed:28356425]
Clin Cancer Res. 2017 Aug 1;23(15):4066-4076.
Purpose: We aimed to establish the MTD of the poly (ADP-ribose) (PAR) polymerase inhibitor, veliparib, in combination with Carboplatin in germline BRCA1- and BRCA2- (BRCA)-associated metastatic breast cancer (MBC), to assess the efficacy of single-agent veliparib, and of the combination treatment after progression, and to correlate PAR levels with clinical outcome.Experimental Design: Phase I patients received Carboplatin (AUC of 5-6, every 21 days), with escalating doses (50-20 mg) of oral twice-daily (BID) veliparib. In a companion phase II trial, patients received single-agent veliparib (400 mg BID), and upon progression, received the combination at MTD. Peripheral blood mononuclear cell PAR and serum veliparib levels were assessed and correlated with outcome.Results: Twenty-seven phase I trial patients were evaluable. Dose-limiting toxicities were nausea, dehydration, and thrombocytopenia [MTD: veliparib 150 mg po BID and Carboplatin (AUC of 5)]. Response rate (RR) was 56%; 3 patients remain in complete response (CR) beyond 3 years. Progression-free survival (PFS) and overall survival (OS) were 8.7 and 18.8 months. The PFS and OS were 5.2 and 14.5 months in the 44 patients in the phase II trial, with a 14% RR in BRCA1 (n = 22) and 36% in BRCA2 (n = 22). One of 30 patients responded to the combination therapy after progression on veliparib. Higher baseline PAR was associated with clinical benefit.Conclusions: Safety and efficacy are encouraging with veliparib alone and in combination with Carboplatin in BRCA-associated MBC. Lasting CRs were observed when the combination was administered first in the phase I trial. Further investigation of PAR level association with clinical outcomes is warranted. Clin Cancer Res; 23(15); 4066-76. (c)2017 AACR.
An Open-Label, Randomized, Controlled Phase II Study of Paclitaxel-Carboplatin Chemotherapy With Necitumumab Versus Paclitaxel-Carboplatin Alone in First-Line Treatment of Patients With Stage IV Squamous Non-Small-Cell Lung Cancer.[Pubmed:28365238]
Clin Lung Cancer. 2017 Sep;18(5):480-488.
BACKGROUND: The combination of necitumumab with gemcitabine-cisplatin significantly improved overall survival (OS) in patients with stage IV squamous non-small-cell lung cancer (NSCLC), in the phase III SQUamous NSCLC treatment with the Inhibitor of EGF REceptor (SQUIRE) trial. Paclitaxel-Carboplatin was selected as an alternative standard of care in the current phase II study. PATIENTS AND METHODS: Patients were randomized (stratified according to Eastern Cooperative Oncology Group performance status and sex) 2:1 to Carboplatin with or without necitumumab. Chemotherapy was paclitaxel 200 mg/m(2) on day 1 Q3W and Carboplatin area under the curve 6 on day 1 Q3W. Necitumumab 800 mg, on days 1 and 8, was continued until disease progression or intolerable toxicity occurred. The primary end point was objective response rate (ORR) on the basis of Response Evaluation Criteria In Solid Tumors version 1.1. RESULTS: One hundred sixty-seven patients were randomized to the necitumumab-containing arm (n = 110) or the chemotherapy-only arm (n = 57). The combination of necitumumab with chemotherapy resulted in an ORR of 48.9% versus 40.0%. Median progression-free survival and OS were 5.4 versus 5.6 months (hazard ratio [HR], 1.0) and 13.2 versus 11.2 months (HR, 0.83; P = .379) in each treatment arm, respectively. Disease control rate was 87.2% versus 84.0%. Grade >/= 3 adverse events typically associated with epidermal growth factor receptor (EGFR) monoclonal antibodies showing a > 2% increase were hypomagnesemia (5.7% vs. 0) and rash (2.8% vs. 0). Any Grade thromboembolic events occurred in < 4% of patients in either arm. CONCLUSION: The results of our study support previously reported results that the combination of necitumumab with chemotherapy improves survival in patients with advanced squamous NSCLC and shows a safety profile consistent with that of EGFR monoclonal antibodies.
Long-term Outcomes of Induction Carboplatin and Gemcitabine Followed by Concurrent Radiotherapy With Low-dose Paclitaxel and Gemcitabine for Stage III Non-small-cell Lung Cancer.[Pubmed:28344046]
Clin Lung Cancer. 2017 Sep;18(5):565-571.
BACKGROUND: Standard treatment for unresectable stage III non-small-cell lung cancer (NSCLC) is concurrent chemo-radiation (CRT). A regimen of induction Carboplatin and gemcitabine followed by CRT was developed at the McGill University Health Centre to prevent delays in treatment initiation. We report the long-term outcomes with this regimen based on a pooled analysis of both protocol patients from a phase II study and nonprotocol patients. METHODS AND MATERIALS: Outcomes and toxicity data were retrieved for 142 patients with stage III NSCLC: 43 patients treated on protocol between January 2003 and November 2004, and 101 patients treated off-protocol between December 2004 and August 2013. Patients received 2 cycles of Carboplatin with an area under the curve of 5 intravenously (IV) on day 1 and gemcitabine 1000 mg/m(2) IV on days 1 and 8 every 3 weeks, followed on day 50 by CRT, 60 Gy/30 over 6 weeks, concomitantly with 2 cycles of paclitaxel 50 mg/m(2) IV and gemcitabine 100 mg/m(2) IV on days 1 and 8 every 3 weeks. RESULTS: The median overall survival was 23.2 months. With a median follow-up of 23.8 months, the 3-, 4-, and 5-year overall survival was 38%, 30%, and 26%, respectively. The median and 5-year progression-free survival rates were 12.5 months and 25%, respectively. Rates of grade >/= 3 hematologic, esophageal, and respiratory toxicity were 20%, 10%, and 10%, respectively. Forty-eight patients received further lines of chemotherapy. CONCLUSION: The present analysis affirms the favorable toxicity profile of this novel induction chemotherapy, without apparent compromise in clinical outcomes, when compared with regimens using immediate concurrent CRT.
Comparison of Hypersensitivity Reactions to Carboplatin Retreatment in Gynecologic Cancer Patients between One and Two Hour Infusions: a Randomized Trial Study[Pubmed:28345825]
Asian Pac J Cancer Prev. 2017 Feb 1;18(2):425-430.
Objective: To compare the incidence rate of Carboplatin hypersensitivity reactions (HSRs) in gynecologic cancer patients receiving one-hour or two-hour Carboplatin retreatment infusions. Setting: A Prospective Randomized Controlled Trial. Methods: Recurrent gynecologic cancer patients 25 to 80-years of age who were scheduled to receive Carboplatin retreatment after previously receiving at least six cycles of Carboplatin without a history of platinum allergy were invited to enroll. They were randomized to receive either a one-hour or two-hour Carboplatin infusion in each cycle. The nurses recorded any occurrence of HSR. Patients who developed Carboplatin HSR were discontinued from the study. Results: Forty-five patients were enrolled and randomized to receive either a one-hour Carboplatin infusion arm in 69 cycles or a two-hour infusion arm in 67 cycles. Both groups were well balanced regarding median age, body mass index, type of cancer, history of drug allergy, median platinum free interval time, median total number of previous Carboplatin cycles, premedication type, regimen and median total dose of Carboplatin. Five (3.67%) of the 136 cycles resulted in Carboplatin HSR, all of which were Grade 1. Of these, four cycles developed HSR during the one-hour infusion and only one cycle with a two-hour infusion (P=0.37). The onset of Carboplatin HSR occurred within 30-105 minutes after infusion start. Conclusion: Extending the Carboplatin infusion time to two hours from one hour did not significantly decrease Carboplatin HSR.
High accumulation of platinum-DNA adducts in strial marginal cells of the cochlea is an early event in cisplatin but not carboplatin ototoxicity.[Pubmed:16569706]
Mol Pharmacol. 2006 Jul;70(1):23-9.
Ototoxicity is a typical dose-limiting side effect of cancer chemotherapy with cisplatin but much less so with Carboplatin. To elucidate the underlying molecular pathological mechanisms, we have measured the formation and persistence of drug-induced DNA adducts in the nuclei of inner ear cells of guinea pigs after short-term exposure to either cisplatin or Carboplatin using immunofluorescence staining and quantitative image analysis. After application of Carboplatin, all cells of the cochlea exhibited a similar burden of guanine-guanine intrastrand cross-links in DNA. In contrast, we observed a pronounced 3- to 5-fold accumulation of this cytotoxic adduct exclusively in the marginal cells of the stria vascularis between 8 and 48 h after treatment with cisplatin. In the kidney, the other critical target tissue of cisplatin toxicity, a similar high preferential formation of cytotoxic DNA adducts was measured in the tubular epithelial cells but not in other renal cell types. As for the ear, this excessive formation of DNA damage in a particular cell type was seen in animals treated with cisplatin but not those treated with Carboplatin. Because cisplatin ototoxicity is often attributed to oxidative stress mediated by the generation of radical oxygen species (ROS), we have measured in parallel the levels of the lead DNA oxidation product 8-oxoguanine (8-oxoG) in cochlear cryosections. Compared with basal levels in untreated control cochleas, no additional formation of 8-oxoG was detectable up to 48 h after cisplatin treatment in the DNA of either inner-ear cell type. This suggests that the generation of ROS may be a secondary event in cisplatin ototoxicity.
Increased DNA-binding activity of cis-1,1-cyclobutanedicarboxylatodiammineplatinum(II) (carboplatin) in the presence of nucleophiles and human breast cancer MCF-7 cell cytoplasmic extracts: activation theory revisited.[Pubmed:10535754]
Biochem Pharmacol. 1999 Nov 15;58(10):1625-9.
The molecular mechanism of Carboplatin [cis-1,1-cyclobutanedicarboxylatodiammineplatinum(II)] activation is still unresolved. We studied the binding of Carboplatin to calf thymus DNA in the presence of thiourea, glutathione, and human breast cancer MCF-7 cell cytoplasmic extracts by measurement of DNA-dependent ethidium bromide fluorescence and atomic absorption spectroscopy. After a 96-hr period of reaction, the decrease in the DNA-dependent fluorescence yield of ethidium bromide due to the formation of platinum (Pt)-DNA adducts increased significantly in the presence of thiourea (6-fold) and glutathione (3- to 4-fold) as compared to the controls in the absence of the nucleophiles. There was also a marked elevation in the levels of platinum incorporated into DNA, measured by atomic absorption spectroscopy (2- to 3-fold and 5- to 7-fold for thiourea and glutathione, respectively). More remarkably, the Pt-DNA adducts formed in the presence of cytoplasmic extracts of MCF-7 human breast cancer cells also showed similar results in a dose-related fashion. Carboplatin, therefore, displayed a characteristic increase in DNA binding/damaging in the presence of the very same S-containing nucleophiles that showed the expected quenching effects in the case of cisplatin [cis-diamminedichloroplatinum (II)]. We propose a nucleophile-facilitated release of the active species of Carboplatin prior to binding with DNA.


