SN-6Selective Na+/Ca2+ exchange inhibitor (reverse mode) CAS# 415697-08-4 |
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 415697-08-4 | SDF | Download SDF |
| PubChem ID | 10222761 | Appearance | Powder |
| Formula | C20H22N2O5S | M.Wt | 402.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 62.5 mg/mL (155.29 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
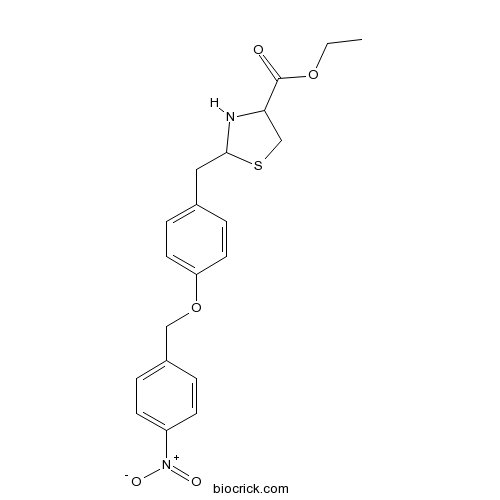
| Chemical Name | ethyl 2-[[4-[(4-nitrophenyl)methoxy]phenyl]methyl]-1,3-thiazolidine-4-carboxylate | ||
| SMILES | CCOC(=O)C1CSC(N1)CC2=CC=C(C=C2)OCC3=CC=C(C=C3)[N+](=O)[O-] | ||
| Standard InChIKey | ZVYIJXLMBWCGHP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H22N2O5S/c1-2-26-20(23)18-13-28-19(21-18)11-14-5-9-17(10-6-14)27-12-15-3-7-16(8-4-15)22(24)25/h3-10,18-19,21H,2,11-13H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective Na+/Ca2+-exchange (NCX) inhibitor; displays some selectivity for NCX1. IC50 values are 2.9, 16 and 8.6 μM for inhibition of intracellular Na+-dependent 45Ca2+ uptake by cells expressing NCX1, NCX2 and NCX3 respectively. Has some affinity for mACh receptors (IC50 = 18 μM) but minimal activity against NCKX2 and various receptors and ion channels (IC50 > 30 μM). Preferentially blocks Ca2+ influx mode and is more selective for NCX isoforms than KB-R7943. Anti-ischemic; potently protects against hypoxia-induced renal tubular cell damage (IC50 = 0.63 μM). |

SN-6 Dilution Calculator

SN-6 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4845 mL | 12.4224 mL | 24.8447 mL | 49.6894 mL | 62.1118 mL |
| 5 mM | 0.4969 mL | 2.4845 mL | 4.9689 mL | 9.9379 mL | 12.4224 mL |
| 10 mM | 0.2484 mL | 1.2422 mL | 2.4845 mL | 4.9689 mL | 6.2112 mL |
| 50 mM | 0.0497 mL | 0.2484 mL | 0.4969 mL | 0.9938 mL | 1.2422 mL |
| 100 mM | 0.0248 mL | 0.1242 mL | 0.2484 mL | 0.4969 mL | 0.6211 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N6-Cyclopentyladenosine
Catalog No.:BCC7160
CAS No.:41552-82-3
- 1-(3,4-dimethoxyphenyl)-2-(4-allly-2,6-dimethoxyphenoxy)propan-1-ol
Catalog No.:BCN1445
CAS No.:41535-95-9
- 2,6,16-Kauranetriol
Catalog No.:BCN5472
CAS No.:41530-90-9
- Koaburaside monomethyl ether
Catalog No.:BCN5471
CAS No.:41514-64-1
- Daturaolone
Catalog No.:BCN3904
CAS No.:41498-80-0
- 12-Oleanene-3,6-diol
Catalog No.:BCN3903
CAS No.:41498-79-7
- 3-Amino-1-phenyl-2-pyrazolin-5-one
Catalog No.:BCC8605
CAS No.:4149-06-8
- Belinostat (PXD101)
Catalog No.:BCC2153
CAS No.:414864-00-9
- Turkesterone
Catalog No.:BCN2363
CAS No.:41451-87-0
- Bruceantin
Catalog No.:BCN7618
CAS No.:41451-75-6
- Onetine
Catalog No.:BCN2102
CAS No.:41451-67-6
- Phalaenopsine Is
Catalog No.:BCN2016
CAS No.:41451-64-3
- RI-1
Catalog No.:BCC1896
CAS No.:415713-60-9
- Poricoic acid G
Catalog No.:BCN8267
CAS No.:415724-84-4
- Carboplatin
Catalog No.:BCC1170
CAS No.:41575-94-4
- 4,R-ajmalicine N-oxide
Catalog No.:BCN5473
CAS No.:41590-29-8
- (-)-Pinoresinol 4-O-glucoside
Catalog No.:BCN7251
CAS No.:41607-20-9
- Ylangenol
Catalog No.:BCN6705
CAS No.:41610-69-9
- Ciclopirox ethanolamine
Catalog No.:BCC4372
CAS No.:41621-49-2
- Lacinilene C
Catalog No.:BCN5474
CAS No.:41653-72-9
- Koaburaside
Catalog No.:BCN5475
CAS No.:41653-73-0
- 7-O-Methyl morroniside
Catalog No.:BCN3882
CAS No.:41679-97-4
- Catharanthine hemitartrate
Catalog No.:BCN8463
CAS No.:4168-17-6
- 2H-1-Benzopyran-7-yloxy
Catalog No.:BCN3580
CAS No.:41680-08-4
TbNb(6)Sn(6): the first ternary compound from the rare earth-niobium-tin system.[Pubmed:21589205]
Acta Crystallogr Sect E Struct Rep Online. 2010 Nov 17;66(Pt 12):i82.
The title compound, terbium hexa-niobium hexastannide, TbNb(6)Sn(6), is the first ternary compound from the rare earth-niobium-tin system. It has the HfFe(6)Ge(6) structure type, which can be analysed as an inter-growth of the Zr(4)Al(3) and CaCu(5) structures. All the atoms lie on special positions; their coordination geometries and site symmetries are: Tb (dodeca-hedron) 6/mmm; Nb (distorted icosa-hedron) 2mm; Sn (Frank-Caspar polyhedron, CN = 14-15) 6mm and m2; Sn (distorted icosa-hedron) m2. The structure contains a graphite-type Sn network, Kagome nets of Nb atoms, and Tb atoms alternating with Sn2 dumbbells in the channels.
Amiloride and SN-6 suppress audiogenic seizure susceptibility in genetically epilepsy-prone rats.[Pubmed:24948133]
CNS Neurosci Ther. 2014 Sep;20(9):860-6.
AIMS: We have recently reported that amiloride, a potent and nonselective blocker of acid-sensing ion channels, prevents the development of pilocarpine-induced seizures and status epilepticus. Amiloride is also known to suppress the activity of Na(+) /Ca(2+) and Na(+) /H(+) exchangers that have been implicated in the pathophysiology of seizures. Here, we evaluated the effects of amiloride, SN-6 (a potent blocker of Na(+) /Ca(2+) exchangers) and zoniporide (a potent blocker of Na(+) /H(+) exchangers) on acoustically evoked seizures (audiogenic seizures, AGS) in genetically epilepsy-prone rats (GEPR-3s), a model of inherited generalized epilepsy. METHODS: Male, six-week-old GEPR-3s were used. The GEPR-3s were tested for AGS susceptibility before and after treatment with various doses of amiloride, SN-6, and zoniporide (1, 3, 10, and 30 mg/kg; per os). RESULTS: We found that pretreatment with amiloride and SN-6 markedly reduced the incidence and severity of AGS in the GEPR-3s. In contrast, administration of zoniporide only minimally reduced the incidence and severity of AGS in the GEPR-3s. A combination of noneffective doses of SN-6 and zoniporide also suppressed AGS susceptibility in the GEPR-3s. CONCLUSIONS: These findings suggest acid-sensing ion channels and the Na(+) /Ca(2+) exchanger may play an important role in the pathophysiology of inherited AGS susceptibility in the GEPR-3s.
The effect of SN-6, a novel sodium-calcium exchange inhibitor, on contractility and calcium handling in isolated failing rat ventricular myocytes.[Pubmed:24106913]
Cardiovasc Ther. 2013 Dec;31(6):e115-24.
BACKGROUND AND PURPOSE: Specific Na(+) /Ca(2+) exchanger (NCX) inhibition is a potential strategy to correct reduced contractility and depleted sarcoplasmic reticulum (SR) Ca(2+) content in heart failure (HF). SN-6, a benzyloxyphenyl derivative and proposed selective NCX inhibitor, could be used for this purpose. This study aimed to evaluate the effects of SN-6 on contractility and Ca(2+) handling in normal and failing rat cardiomyocytes. EXPERIMENTAL APPROACH: HF was induced in rats by coronary artery ligation. Left ventricular myocytes were isolated and superfused with increasing concentrations of SN-6. KEY RESULTS: Sarcomere shortening, induced by field-stimulation, was reduced in amplitude with increasing concentrations of SN-6 compared with control solution. This effect was greater in failing cells. Kinetics of contractility (time to 90% peak and time to 50% relaxation) were significantly faster. Despite this, intracellular Ca(2+) transients demonstrated no change in the peak amplitude at low concentrations of SN-6, suggesting that SN-6 may affect myofilament sensitivity to Ca(2+) . Ten micro molar SN-6 significantly reduced peak Ca(2+) amplitude by 61.57% and 64.73% in normal and failing cells, respectively. Diastolic Ca(2+) was significantly increased at 1 muM SN-6. SR Ca(2+) content, assessed by rapid application of caffeine, was reduced in failing cells with 1 muM SN-6. Peak ICa , measured by whole-cell patch clamping, was significantly reduced in normal and failing myocytes at 1 muM SN-6. CONCLUSIONS AND IMPLICATIONS: Our data suggest that SN-6 is not a selective inhibitor of NCX and impairs contractility and Ca(2+) handling. Its use, together with similar putative NCX blockers, in correcting the contractile abnormalities of heart failure requires further studies.
Na6ZnSn2, Na4.24K1.76(1)ZnSn2, and Na20Zn8Sn11: three intermetallic structures containing the linear {Sn-Zn-Sn}6- unit.[Pubmed:19138150]
J Am Chem Soc. 2009 Feb 4;131(4):1469-78.
The novel intermetallic compounds Na6ZnSn2 (1), Na4.24K1.76(1)ZnSn2 (2), and Na20Zn8Sn11 (3) were obtained from direct fusion of the pure elements, and their structures were determined by single crystal X-ray diffraction. All three compounds adopt new structure types and contain linear anionic {Sn-Zn-Sn}6- units with rather short Zn-Sn contacts (2.55-2.58 A), separated by alkali metal counterions. Compound 3 comprises layers of interconnected heteroatomic {Zn7Sn5} icosahedra as an additional unique structural motif. The bonding situation in this 16 valence-electron anion is analyzed by quantum chemical methods. The results of NBO, AIM, and ELF calculations (Gaussian03 on HF/3-21G level) reveal covalent bonding between Sn and Zn. The relationship to isovalent CO2 is discussed. Band structure calculations on the density functional theory level (LMTO) show that 1 can be understood as a Zintl phase containing a {Sn-Zn-Sn}6- anion; however, Na-Sn contacts must also be considered. Magnetic susceptibility measurements show a temperature-independent, weak diamagnetism for Na6ZnSn2 (1).
The exchanger inhibitory peptide region-dependent inhibition of Na+/Ca2+ exchange by SN-6 [2-[4-(4-nitrobenzyloxy)benzyl]thiazolidine-4-carboxylic acid ethyl ester], a novel benzyloxyphenyl derivative.[Pubmed:15213295]
Mol Pharmacol. 2004 Jul;66(1):45-55.
We investigated the properties and interaction domains of SN-6 [2-[4-(4-nitrobenzyloxy)benzyl]thiazolidine-4-carboxylic acid ethyl ester], a newly synthesized and selective Na(+)/Ca(2+) exchange (NCX) inhibitor. SN-6 (0.3-30 microM) inhibited preferentially intracellular Na(+)-dependent (45)Ca(2+) uptake (i.e., the reverse mode) compared with extracellular Na(+)-dependent (45)Ca(2+) efflux (i.e., the forward mode) in NCX1-transfected fibroblasts. SN-6 was 3- to 5-fold more inhibitory to (45)Ca(2+) uptake in NCX1 (IC(50) = 2.9 microM) than to that in NCX2 or NCX3 but not to that in NCKX2. We searched for regions that may form the SN-6 receptor by NCX1/NCX3-chimeric analyses and determined that amino acid regions 73 to 108 and 193 to 230 in NCX1 are mostly responsible for the differential drug response between NCX1 and NCX3. Further site-directed mutagenesis revealed that double substitutions of Val227 and Tyr228 in NCX1, which exist within the exchanger inhibitory peptide (XIP) region, mimicked the different drug response. In addition, F213R, G833C, and N839A mutations in NCX1 resulted in loss of drug sensitivity. Exchangers with mutated XIP regions, which display either undetectable or accelerated Na(+)-dependent inactivation, had markedly reduced sensitivity or hypersensitivity to SN-6, respectively. Cell ATP depletion enhanced the inhibitory potency of SN-6. Therefore, SN-6 at lower doses (IC(50) = 0.63 microM) potently protected against hypoxia/reoxygenation-induced cell damage in renal tubular cells overexpressing NCX1, suggesting that this drug predominantly works under hypoxic/ischemic conditions. These properties of SN-6, which may be derived from its interaction with the XIP region, are advantageous to developing it as a new anti-ischemic drug.
Forefront of Na+/Ca2+ exchanger studies: molecular pharmacology of Na+/Ca2+ exchange inhibitors.[Pubmed:15359084]
J Pharmacol Sci. 2004 Sep;96(1):27-32. Epub 2004 Sep 10.
The Na+/Ca2+ exchanger (NCX) is an ion transporter that exchanges Na+ and Ca2+ in either Ca2+ efflux or Ca2+ influx mode, depending on membrane potential and transmembrane ion gradients. In myocytes, neurons, and nephron cells, NCX is thought to play an important role in the regulation of intracellular Ca2+ concentration. Recently, the benzyloxyphenyl derivatives KB-R7943, SEA0400, and SN-6 have been developed as selective NCX inhibitors. Currently, SEA0400 is the most potent and selective inhibitor. These inhibitors possess different isoform-selectivities, although they have similar properties, such as Ca2+ influx mode-selectivity and I1 inactivation-dependence. Recent site-directed mutagenesis has revealed that these inhibitors possess some molecular determinants (Phe-213, Val-227, Tyr-228, Gly-833, and Asn-839) for interaction with NCX1. These benzyloxyphenyl derivatives are expected to be useful tools to study the physiological roles of NCX. Moreover, such inhibitors may have therapeutic potential as a new remedy for ischemic disease, arrhythmias, heart failure, and hypertension.


