BruceantinCAS# 41451-75-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 41451-75-6 | SDF | Download SDF |
| PubChem ID | 5281304 | Appearance | Powder |
| Formula | C28H36O11 | M.Wt | 548.57 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Synonyms | (-)-Bruceantin; NCI165563; NSC165563 | ||
| Solubility | Soluble in DMSO | ||
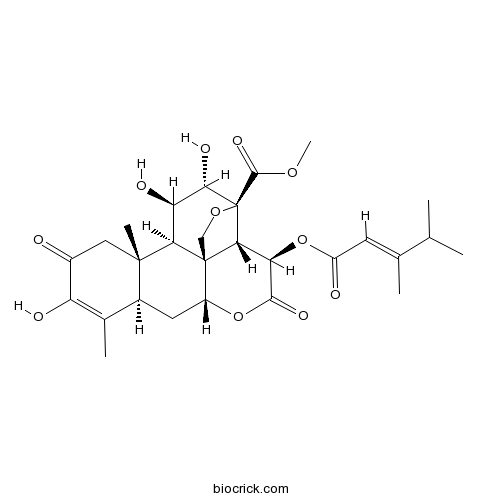
| SMILES | CC1=C(C(=O)CC2(C1CC3C45C2C(C(C(C4C(C(=O)O3)OC(=O)C=C(C)C(C)C)(OC5)C(=O)OC)O)O)C)O | ||
| Standard InChIKey | IRQXZTBHNKVIRL-GOTQHHPNSA-N | ||
| Standard InChI | InChI=1S/C28H36O11/c1-11(2)12(3)7-17(30)39-20-22-27-10-37-28(22,25(35)36-6)23(33)19(32)21(27)26(5)9-15(29)18(31)13(4)14(26)8-16(27)38-24(20)34/h7,11,14,16,19-23,31-33H,8-10H2,1-6H3/b12-7+/t14-,16+,19+,20+,21+,22+,23-,26-,27+,28-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Bruceantin has antiviral activity, it can inhibit pepper mottle virus in pepper. 2. Bruceantin is an effective agent in controlling the proliferation, viability and migration of multiple myeloma cancer stem cells (CSCs) as well as angiogenesis in vitro. 3. Bruceantin exhibits NF-κB p65 inhibition, and cytotoxic potential against HT-29, HeLa, and HL-60 cells . 4. Bruceantin shows high antimalarial activity. |
| Targets | Antifection | p65 | NF-kB | ROS |

Bruceantin Dilution Calculator

Bruceantin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8229 mL | 9.1146 mL | 18.2292 mL | 36.4584 mL | 45.573 mL |
| 5 mM | 0.3646 mL | 1.8229 mL | 3.6458 mL | 7.2917 mL | 9.1146 mL |
| 10 mM | 0.1823 mL | 0.9115 mL | 1.8229 mL | 3.6458 mL | 4.5573 mL |
| 50 mM | 0.0365 mL | 0.1823 mL | 0.3646 mL | 0.7292 mL | 0.9115 mL |
| 100 mM | 0.0182 mL | 0.0911 mL | 0.1823 mL | 0.3646 mL | 0.4557 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bruceantin(NSC165563) is first isolated from Brucea antidysenterica, a tree used in Ethiopia for the treatment of cancer, and activity was observed against B16 melanoma, colon 38, and L1210 and P388 leukemia in mice. IC50 value: Target: anticancer Cell differentiation was induced and c-MYC was down-regulated, suggesting a mechanistic correlation between c-MYC down-regulation and induction of cell differentiation or cell death. Treatment of HL-60 and RPMI 8226 cell lines induced apoptosis, and this involved the caspase and mitochondrial pathways. Moreover, an in vivo study using RPMI 8226 human-SCID xenografts demonstrated that bruceantin induced regression in early as well as advanced tumors, and these significant antitumor responses were facilitated in the absence of overt toxicity.
References:
[1]. Cuendet M, et al. Multiple myeloma regression mediated by bruceantin. Clin Cancer Res. 2004 Feb 1;10(3):1170-9.
[2]. Cuendet M, et al. Antitumor activity of bruceantin: an old drug with new promise. J Nat Prod. 2004 Feb;67(2):269-72.
- Onetine
Catalog No.:BCN2102
CAS No.:41451-67-6
- Phalaenopsine Is
Catalog No.:BCN2016
CAS No.:41451-64-3
- (+)-N-Methylallosedridine
Catalog No.:BCN5470
CAS No.:41447-16-9
- (-)-N-Methylsedridine
Catalog No.:BCN5469
CAS No.:41447-15-8
- ODQ
Catalog No.:BCC6829
CAS No.:41443-28-1
- 5-Heptadecylresorcinol
Catalog No.:BCN4750
CAS No.:41442-57-3
- 8-Hydroxyapigenin
Catalog No.:BCN8404
CAS No.:41440-05-5
- 5-Benzoylpentanoic acid
Catalog No.:BCC8740
CAS No.:4144-62-1
- Cirsilineol
Catalog No.:BCN2560
CAS No.:41365-32-6
- Stachydrine hydrochloride
Catalog No.:BCN5332
CAS No.:4136-37-2
- 1,3,6-Trihydroxy-5-methoxyxanthone
Catalog No.:BCN3454
CAS No.:41357-84-0
- Anhydrotuberosin
Catalog No.:BCN5468
CAS No.:41347-49-3
- Turkesterone
Catalog No.:BCN2363
CAS No.:41451-87-0
- Belinostat (PXD101)
Catalog No.:BCC2153
CAS No.:414864-00-9
- 3-Amino-1-phenyl-2-pyrazolin-5-one
Catalog No.:BCC8605
CAS No.:4149-06-8
- 12-Oleanene-3,6-diol
Catalog No.:BCN3903
CAS No.:41498-79-7
- Daturaolone
Catalog No.:BCN3904
CAS No.:41498-80-0
- Koaburaside monomethyl ether
Catalog No.:BCN5471
CAS No.:41514-64-1
- 2,6,16-Kauranetriol
Catalog No.:BCN5472
CAS No.:41530-90-9
- 1-(3,4-dimethoxyphenyl)-2-(4-allly-2,6-dimethoxyphenoxy)propan-1-ol
Catalog No.:BCN1445
CAS No.:41535-95-9
- N6-Cyclopentyladenosine
Catalog No.:BCC7160
CAS No.:41552-82-3
- SN-6
Catalog No.:BCC7273
CAS No.:415697-08-4
- RI-1
Catalog No.:BCC1896
CAS No.:415713-60-9
- Poricoic acid G
Catalog No.:BCN8267
CAS No.:415724-84-4
Synthesis of A/B-ring partial analogs of bruceantin as potential antimalarial agents.[Pubmed:16789880]
Med Chem. 2005 Jan;1(1):3-11.
Bruceantin (1), a classical quassinoid with the highest reported antimalarial activity among the quassinoids examined thus far, was selected as a natural product lead for the design of a series of A/B-ring analogs. A viable strategy for the synthesis of the series was developed. The functionalized A-ring and the C-15 ester moiety in Bruceantin are incorporated in all designed compounds. The preliminary bioassay results will be discussed in detail.
NF-kappaB inhibitors from Brucea javanica exhibiting intracellular effects on reactive oxygen species.[Pubmed:20944100]
Anticancer Res. 2010 Sep;30(9):3295-300.
AIM: Brucea javanica was studied to identify nuclear factor kappaB (NF-kappaB) inhibitors exhibiting reactive oxygen species (ROS) intracellular amplification. MATERIAL AND METHODS: Eight compounds were evaluated for selective cytotoxicity using HT-29, HeLa, and HL-60 cells, and in a NF-kappaB assay. Active compounds were then tested using ROS and mitochondria transmembrane potential (MTP) assays. NF-kappaB and nuclear factor activated T-cell (NFAT) translocation were also assessed using their respective whole cell assays. RESULTS: Bruceajavanone B, Bruceantin, bruceine A, (-)-hydnocarpin, and chrysoeriol exhibited cytotoxic potential and NF-kappaB p65 inhibition. Chrysoeriol exhibited selective cytotoxicity against leukemia cells with greater potency and also showed an ability to up-regulate NFAT transcriptional pathways through the amplification of intracellular ROS, in the presence of H2O2, to a greater degree than Bruceantin and bruceine. CONCLUSION: Chrysoeriol selectively kills leukemic cells and potentiates the amplification of ROS levels. Therefore, chrysoeriol could serve as a potential chemotherapeutic modifier for leukemia chemotherapy since leukemia cells have a higher susceptibility to elevated ROS levels.
Bruceantin inhibits multiple myeloma cancer stem cell proliferation.[Pubmed:27434731]
Cancer Biol Ther. 2016 Sep;17(9):966-75.
Multiple myeloma (MM) continues to claim the lives of a majority of patients. MM cancer stem cells (CSCs) have been demonstrated to sustain tumor growth. Due to their ability to self-renew and to express detoxifying enzymes and efflux transporters, MM-CSCs are rendered highly resistant to conventional therapies. Therefore, managing MM-CSCs characteristics could have profound clinical implications. Bruceantin (BCT) is a natural product previously demonstrated to inhibit the growth of MM in RPMI 8226 cells-inoculated mouse xenograft models, and to cause regression in already established tumors. The objectives of the present study were to test the inhibitory effects of BCT on MM-CSCs growth derived from a human primary tumor, and to explore a mechanism of action underlying these effects. BCT exhibited potent antiproliferative activity in MM-CSCs starting at 25 nM. BCT induced cell cycle arrest, cell death and apoptosis in MM-CSCs as well as inhibited cell migration and angiogenesis in vitro. Using a qPCR screen, it was found that the gene expression of a number of Notch pathway members was altered. Pretreatment of MM-CSCs with the gamma-secretase inhibitor RO4929097, a Notch pathway inhibitor, reversed BCT-induced effects on MM-CSCs proliferation. In this study, BCT was shown to be an effective agent in controlling the proliferation, viability and migration of MM-CSCs as well as angiogenesis in vitro. The effect on MM-CSCs proliferation may be mediated by the Notch pathway. These results warrant further investigation of BCT in a broader set of human-derived MM-CSCs and with in vivo models representative of MM.
Quassinoids isolated from Brucea javanica inhibit pepper mottle virus in pepper.[Pubmed:27686478]
Virus Res. 2017 Jan 2;227:49-56.
A green fluorescent protein (GFP)-tagged pepper mottle virus (PepMoV) based leaf-disc method and systemic host method were developed to identify antiviral agents. Preliminary experiments using a PepMoV-GFP based leaf-disc method led to the isolation of five quassinoids, including brusatol (1), Bruceantin (2), brucein A (3), Bruceantinol (4), and brucein B (5), from the CH3OH extract of Brucea javanica. All isolated compounds exhibited inactivation effects in systemic host plants, and compounds 3 and 4 were potent, with a minimum inhibitory concentration of 10muM. Furthermore, compound 3 was found to have a protective effect at the tested concentration of 40muM.


