Belinostat (PXD101)Hydroxamate-type HDAC inhibitor CAS# 414864-00-9 |
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- NSC 3852
Catalog No.:BCC2423
CAS No.:3565-26-2
- LAQ824 (NVP-LAQ824,Dacinostat)
Catalog No.:BCC2160
CAS No.:404951-53-7
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
- KD 5170
Catalog No.:BCC2420
CAS No.:940943-37-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 414864-00-9 | SDF | Download SDF |
| PubChem ID | 6918638 | Appearance | Powder |
| Formula | C15H14N2O4S | M.Wt | 318.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 64 mg/mL (201.03 mM) in DMSO | ||
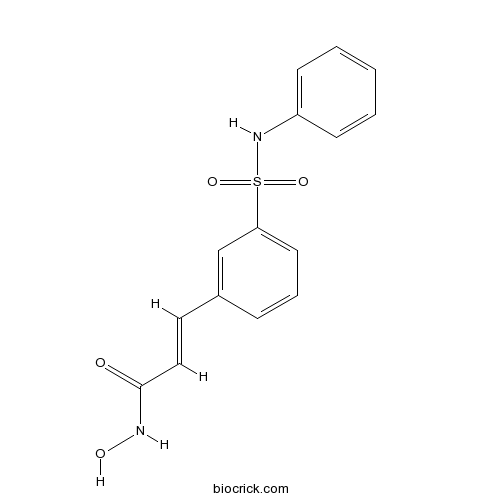
| Chemical Name | (E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2-enamide | ||
| SMILES | C1=CC=C(C=C1)NS(=O)(=O)C2=CC=CC(=C2)C=CC(=O)NO | ||
| Standard InChIKey | NCNRHFGMJRPRSK-MDZDMXLPSA-N | ||
| Standard InChI | InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Belinostat (PXD101) is a novel inhibitor of pan-HDAC with an IC50 value of 27 nM. | |||||
| Targets | pan-HDAC | |||||
| IC50 | 27 nM | |||||
| Kinase experiment [1]: | |
| Inhibitory activities | For activity assays, the reaction was carried out in a total volume of 150 μl of buffer [60 mM Tris (pH 7.4) containing 30% glycerol] containing 2 μl of cell extract and, where used, 2 μl of PXD101. The reaction was started by the addition of 2 μl of [3H]labeled substrate (acetylated histone H4 peptide corresponding to the 20 NH2-terminal residues). Samples were incubated at 37℃ for 45 min, and the reaction stopped by the addition of HCl and acetic acid (0.72 and 0.12 M final concentrations, respectively). Released [3H]acetate was extracted into 750 μl of ethyl acetate, and samples were centrifuged at 12,000×g for 5 min. The upper phase (600 μl) was transferred to 3 ml of scintillation fluid and counted. |
| Cell experiment [2]: | |
| Cell lines | The human urinary bladder carcinoma cell lines 5637, T24, J82 and RT4. |
| Preparation method | Soluble in DMSO > 10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
| Reacting condition | 1-5 μM; 48 h. |
| Applications | In human urinary bladder carcinoma cell lines, belinostat (PXD101) inhibits cell proliferation in a dose dependent way with IC50 values of 1.0, 3.5, 6.0 and 10.0 μM in 5637, T24, J82 and RT4 cell lines, respectively. Belinostat (PXD101) (5 μM) decreases in cell growth and proliferation by 71%, 51%, 41% and 23% in 5637, T24, J82 and RT4 cell lines, respectively. Also, belinostat reduces cells in the S phase and increases cells in the G0-G1 phase. |
| Animal experiment [2]: | |
| Animal models | UPII-Ha-ras transgenic mice. |
| Dosage form | 100 mg/kg; 5 days each week for 3 weeks; intraperitoneal (IP) injections. |
| Preparation method | Dissolved in L-Arginine to give a final concentration of 20 mg/ml. |
| Application | In UPII-Ha-ras transgenic mice, belinostat reduces the weights of Ras-expressing bladders of the male and female transgenic mice by 50% and 36%, respectively. Belinostat inhibits progression of bladder disease. Belinostat shows no detectable toxicity as evaluated by weight. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1]. Plumb JA, Finn PW, Williams RJ, et al. Pharmacodynamic response and inhibition of growth of human tumor xenografts by the novel histone deacetylase inhibitor PXD101. Mol Cancer Ther, 2003, 2(8): 721-728. [2]. Buckley MT, Yoon J, Yee H, et al. The histone deacetylase inhibitor belinostat (PXD101) suppresses bladder cancer cell growth in vitro and in vivo. J Transl Med, 2007, 5: 49. | |

Belinostat (PXD101) Dilution Calculator

Belinostat (PXD101) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1412 mL | 15.706 mL | 31.412 mL | 62.8239 mL | 78.5299 mL |
| 5 mM | 0.6282 mL | 3.1412 mL | 6.2824 mL | 12.5648 mL | 15.706 mL |
| 10 mM | 0.3141 mL | 1.5706 mL | 3.1412 mL | 6.2824 mL | 7.853 mL |
| 50 mM | 0.0628 mL | 0.3141 mL | 0.6282 mL | 1.2565 mL | 1.5706 mL |
| 100 mM | 0.0314 mL | 0.1571 mL | 0.3141 mL | 0.6282 mL | 0.7853 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Belinostat (also known as PXD101) is a novel and potent hydroxamate-type inhibitor of histone deacetylase (HDAC) that inhibits the activity of HDAC in Hela cell extracts with 50% inhibition concentration IC50 value of 27 nM. Belinostat has been found to significantly increase the acetylation of histones H3 and H4 and exerts cytotoxicity in a wide range of tumor cell lines. In the treatment of urothelial carcinoma cell lines, belinostat dose-dependently inhibits proliferation in 5637, T24, J82 and RT4 cells with IC50 values of 1.0 μM, 3.5 μM, 6.0 μM and 10 μM respectively; while, in prostate cancer cell lines, it inhibits cancer cell growth with IC50 values ranging from 0.5 to 2.5 μM.
Reference
Michael T Buckley, Joanne Yoon, Herman Yee, Luis Chiriboga, Leonard Liebes, Gulshan Ara, Xiaozhong Qian, Dean F Bajorin, Tung-Tien Sun, Xue-Ru Wu and Iman Osman. The histone deacetylase inhibitor belinostat (PXD101) suppresses bladder cancer cell growth in vitro and in vivo. Journal of Translational Medicine 2007; 5:49
Giovanni Luca Gravina, Francesco Marampon, Ilaria Giusti, Eleonora Carosa, Stefania Di Sante, Enrico Ricevuto, Vincenza Dolo, Vincenzo Tombolini, Emmanuele A Jannini and Claudio Festuccia. Differential effects of PXD101 (belinostat) on androgen-dependent and androgen-independent prostate cancer models. International Journal of Oncology 40: 711-720, 2012
Plumb JA, Finn PW, Williams RJ, Bandara MJ, Romero MR, Watkins CJ, La Thangue NB, Brown R. Pharmacodynamic response and inhibition of growth of human tumor xenografts by the novel histone deacetylase inhibitor PXD101. Mol Cancer Ther. 2003 Aug;2(8):721-8.
- Turkesterone
Catalog No.:BCN2363
CAS No.:41451-87-0
- Bruceantin
Catalog No.:BCN7618
CAS No.:41451-75-6
- Onetine
Catalog No.:BCN2102
CAS No.:41451-67-6
- Phalaenopsine Is
Catalog No.:BCN2016
CAS No.:41451-64-3
- (+)-N-Methylallosedridine
Catalog No.:BCN5470
CAS No.:41447-16-9
- (-)-N-Methylsedridine
Catalog No.:BCN5469
CAS No.:41447-15-8
- ODQ
Catalog No.:BCC6829
CAS No.:41443-28-1
- 5-Heptadecylresorcinol
Catalog No.:BCN4750
CAS No.:41442-57-3
- 8-Hydroxyapigenin
Catalog No.:BCN8404
CAS No.:41440-05-5
- 5-Benzoylpentanoic acid
Catalog No.:BCC8740
CAS No.:4144-62-1
- Cirsilineol
Catalog No.:BCN2560
CAS No.:41365-32-6
- Stachydrine hydrochloride
Catalog No.:BCN5332
CAS No.:4136-37-2
- 3-Amino-1-phenyl-2-pyrazolin-5-one
Catalog No.:BCC8605
CAS No.:4149-06-8
- 12-Oleanene-3,6-diol
Catalog No.:BCN3903
CAS No.:41498-79-7
- Daturaolone
Catalog No.:BCN3904
CAS No.:41498-80-0
- Koaburaside monomethyl ether
Catalog No.:BCN5471
CAS No.:41514-64-1
- 2,6,16-Kauranetriol
Catalog No.:BCN5472
CAS No.:41530-90-9
- 1-(3,4-dimethoxyphenyl)-2-(4-allly-2,6-dimethoxyphenoxy)propan-1-ol
Catalog No.:BCN1445
CAS No.:41535-95-9
- N6-Cyclopentyladenosine
Catalog No.:BCC7160
CAS No.:41552-82-3
- SN-6
Catalog No.:BCC7273
CAS No.:415697-08-4
- RI-1
Catalog No.:BCC1896
CAS No.:415713-60-9
- Poricoic acid G
Catalog No.:BCN8267
CAS No.:415724-84-4
- Carboplatin
Catalog No.:BCC1170
CAS No.:41575-94-4
- 4,R-ajmalicine N-oxide
Catalog No.:BCN5473
CAS No.:41590-29-8
A Phase II trial of Belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma.[Pubmed:25404094]
Br J Haematol. 2015 Mar;168(6):811-9.
Belinostat is a pan-histone deacetylase inhibitor with antitumour and anti-angiogenic properties. An open label, multicentre study was conducted in patients with peripheral T-cell lymphoma (PTCL) or cutaneous T-cell lymphoma (CTCL) who failed >/=1 prior systemic therapy and were treated with belinostat (1000 mg/m(2) intravenously x5 d of a 21-d cycle). The primary endpoint was objective response rate (ORR). Patients with PTCL (n = 24) had received a median of three prior systemic therapies (range 1-9) and 40% had stage IV disease. Patients with CTCL (n = 29) had received a median of one prior skin-directed therapy (range 0-4) and four prior systemic therapies (range 1-9); 55% had stage IV disease. The ORRs were 25% (PTCL) and 14% (CTCL). Treatment-related adverse events occurred in 77% of patients; nausea (43%), vomiting (21%), infusion site pain (13%) and dizziness (11%) had the highest incidence. Treatment-related serious adverse events were Grade 5 ventricular fibrillation; Grade 4 thrombocytopenia; Grade 3 peripheral oedema, apraxia, paralytic ileus and pneumonitis; and Grade 2 jugular vein thrombosis. Belinostat monotherapy was well tolerated and efficacious in patients with recurrent/refractory PTCL and CTCL. This trial was registered at www.clinicaltrials.gov as NCT00274651.
A Phase I/II Clinical Trial of Belinostat (PXD101) in Combination with Doxorubicin in Patients with Soft Tissue Sarcomas.[Pubmed:27403082]
Sarcoma. 2016;2016:2090271.
Background. Belinostat is a novel histone deacetylase inhibitor. Primary Objectives. Maximum tolerated dose (MTD) and dose limiting toxicities (DLTs) of belinostat (Bel) in combination with doxorubicin (Dox) in solid tumours (phase I) and response rate (RR) in soft tissue sarcomas (phase II). Methods. Bel was administered as a 30-minute IV infusion on days 1-5 and on day 5 with Dox. The dose escalation schedule was as follows: cohort 1: Bel 600 mg/m(2) and 50 mg/m(2) Dox, cohort 2: Bel 600 mg/m(2) and 75 mg/m(2) Dox, cohort 3: Bel 800 mg/m(2) and 75 mg/m(2) Dox, and cohort 4: Bel 1000 mg/m(2) and 75 mg/m(2) Dox. Results. 41 patients were included (25 in phase I, 16 in phase II). Adverse events were fatigue (95%), nausea (76%), and alopecia (63%). There was one DLT, grade 3 rash/hand and foot syndrome. MTD was Bel 1000 mg/m(2)/d and Dox 75 mg/m(2). Four responses were seen: 2 PR in phase I, RR of 8%; in phase II, 1 PR/1 CR, RR of 13%, and 9 patients (56%) with SD. Conclusion. The combination was well tolerated. Response rate was moderate but median time to progression was 6.0 months (95% CI, 1.6-9.7 months) which is superior to some reports of single-agent Dox.
A phase 2 study of belinostat (PXD101) in patients with relapsed or refractory acute myeloid leukemia or patients over the age of 60 with newly diagnosed acute myeloid leukemia: a California Cancer Consortium Study.[Pubmed:24369094]
Leuk Lymphoma. 2014 Oct;55(10):2301-4.
We performed a phase II study of belinostat in patients with acute myeloid leukemia (AML). In this open label phase II study (NCT00357032), patients with relapsed/refractory AML, or newly diagnosed patients with AML over the age of 60, were eligible. Belinostat was administered intravenously (IV) at a dose of 1000 mg/m(2) daily on days 1-5 of a 21-day cycle until progression or unacceptable toxicity. The primary endpoint was complete response (CR) rate, with secondary endpoints of overall response rate (CR + partial response [PR]), time to treatment failure (TTF), overall survival and safety. Twelve eligible patients with AML were enrolled, of whom six had received at least one prior line of therapy. No CR or PR was seen. Four patients had stable disease for at least five cycles. Grade 3 non-hematological toxicities occurred in four patients. Belinostat as monotherapy has minimal single-agent effect in AML on this dosing schedule.
A phase II study of belinostat (PXD101) in relapsed and refractory aggressive B-cell lymphomas: SWOG S0520.[Pubmed:26758422]
Leuk Lymphoma. 2016 Oct;57(10):2359-69.
Recent advances in diffuse large B-cell lymphomas (DLBCL) have underscored the importance of tumor microenvironment in escaping host anti-tumor responses. One mechanism is loss of major histocompatibility Class II antigens (MHCII) associated with decreased tumor infiltrating T lymphocytes (TIL) and poor survival. Transcription of MHCII is controlled by CIITA which in turn is regulated by histone acetylation. In this study, we hypothesized that HDAC inhibition with belinostat increases MHCII, CIITA expression, TIL and improves patient outcomes. Primary objective was evaluation of toxicity and response. Twenty-two patients were enrolled for the study. Belinostat was well tolerated with mild toxicity. Two partial responses were observed at 5, 13 months after registration for an overall response rate (ORR) (95% CI) of 10.5% (1.3-33.1%), and three patients had stable disease for 4.7, 42.3+, and 68.4 + months with minimum 3-year follow-up. Included correlative studies support the hypothesis and serve as the basis for SWOG S0806 combining vorinostat with R-CHOP.


