LAQ824 (NVP-LAQ824,Dacinostat)HDAC inhibitor,potent and novel CAS# 404951-53-7 |
- CUDC-101
Catalog No.:BCC2149
CAS No.:1012054-59-9
- Valproic acid sodium salt (Sodium valproate)
Catalog No.:BCC2156
CAS No.:1069-66-5
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- Belinostat (PXD101)
Catalog No.:BCC2153
CAS No.:414864-00-9
- Trichostatin A (TSA)
Catalog No.:BCC3605
CAS No.:58880-19-6
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 404951-53-7 | SDF | Download SDF |
| PubChem ID | 6445533 | Appearance | Powder |
| Formula | C22H25N3O3 | M.Wt | 379.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NVP-LAQ824; LAQ824 | ||
| Solubility | DMSO : ≥ 43 mg/mL (113.32 mM) *"≥" means soluble, but saturation unknown. | ||
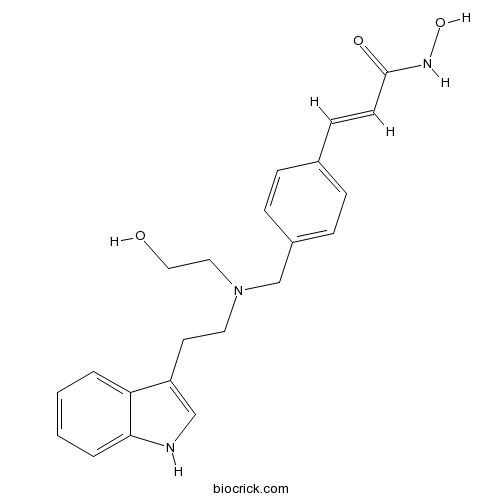
| Chemical Name | (E)-N-hydroxy-3-[4-[[2-hydroxyethyl-[2-(1H-indol-3-yl)ethyl]amino]methyl]phenyl]prop-2-enamide | ||
| SMILES | C1=CC=C2C(=C1)C(=CN2)CCN(CCO)CC3=CC=C(C=C3)C=CC(=O)NO | ||
| Standard InChIKey | BWDQBBCUWLSASG-MDZDMXLPSA-N | ||
| Standard InChI | InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | LAQ824 is a novel inhibitor of HDAC with IC50 value of 32 nM. | |||||
| Targets | HDAC | |||||
| IC50 | 32 nM | |||||
| Cell experiment: [1] | |
| Cell lines | Dexamethasone –sensitive human multiple myeloma (Dex-sensitive MM.1S) cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 20 nM 24-, 48-, or 72-hour |
| Applications | As an inhibitor of HDAC, LAQ824 induces apoptosis in multiple myeloma cell lines resistant to conventional therapies. In this experiment, Dex or LAQ824 alone produced only 15% and 7% growth inhibition, respectively, but produced 51% inhibition when used in combination. |
| Animal experiment : [2] | |
| Animal models | Female BALB/c mice administered 32D.p210 leukemic cells via tail vein injection |
| Dosage form | The mice were given intraperitoneal injection of either 25 mg/kg LAQ824 or D5W vehicle on a daily basis, beginning 3 days after introduction of the leukemic cells. |
| Application | Treatment of mice with LAQ824 delayed the onset of symptoms of leukemia and lethality as compared to mice treated with the vehicle control. Median survival times were 20 days in the LAQ824-treated mice and 15.5 days in the vehicle control mice. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Catley L, Weisberg E, Tai Y T, et al. NVP-LAQ824 is a potent novel histone deacetylase inhibitor with significant activity against multiple myeloma. Blood, 2003, 102(7): 2615-2622. [2] Weisberg E, Catley L, Kujawa J, et al. Histone deacetylase inhibitor NVP-LAQ824 has significant activity against myeloid leukemia cells in vitro and in vivo. Leukemia, 2004, 18(12): 1951-1963. | |

LAQ824 (NVP-LAQ824,Dacinostat) Dilution Calculator

LAQ824 (NVP-LAQ824,Dacinostat) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6353 mL | 13.1766 mL | 26.3532 mL | 52.7065 mL | 65.8831 mL |
| 5 mM | 0.5271 mL | 2.6353 mL | 5.2706 mL | 10.5413 mL | 13.1766 mL |
| 10 mM | 0.2635 mL | 1.3177 mL | 2.6353 mL | 5.2706 mL | 6.5883 mL |
| 50 mM | 0.0527 mL | 0.2635 mL | 0.5271 mL | 1.0541 mL | 1.3177 mL |
| 100 mM | 0.0264 mL | 0.1318 mL | 0.2635 mL | 0.5271 mL | 0.6588 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LAQ824 (also known as NVP-LAQ824 or Dacinostat), a derivative of 4-aminomethylcinnamic hydroxamic acid, is a novel and potent inhibitor of histone deacetylase (HDAC) that inhibits the activity of HDAC with 50% inhibition concentration IC50 value of 0.03 μM. LAQ824 has been found to inhibit the growth of a variety of cancer cell lines, including colon cancer H1299 and HCT116 cells, breast cancer MDA435 cells, prostate cancer DU145 and PC3 cells and non-small cell lung cancer A549 cells, with IC50 value < 1 μM and induce apoptosis in human breast cancer SKBR-3, BT-474 and MB-468 cells. LAQ824 also dose- and time-dependently inhibits the growth of multiple myeloma cells.
Reference
Catley L, Weisberg E, Tai YT, Atadja P, Remiszewski S, Hideshima T, Mitsiades N, Shringarpure R, LeBlanc R, Chauhan D, Munshi NC, Schlossman R, Richardson P, Griffin J, Anderson KC. NVP-LAQ824 is a potent novel histone deacetylase inhibitor with significant activity against multiple myeloma. Blood. 2003 Oct 1;102(7):2615-22. Epub 2003 Jun 19.
- Panobinostat (LBH589)
Catalog No.:BCC3601
CAS No.:404950-80-7
- Ethyl ferulate
Catalog No.:BCN1257
CAS No.:4046-02-0
- Bursehernin
Catalog No.:BCN3040
CAS No.:40456-51-7
- Yatein
Catalog No.:BCN5456
CAS No.:40456-50-6
- Kaempferol-3-O-glucorhamnoside
Catalog No.:BCN2830
CAS No.:40437-72-7
- Pinusolidic acid
Catalog No.:BCN5455
CAS No.:40433-82-7
- erythro-1-Phenylpropane-1,2-diol
Catalog No.:BCN6596
CAS No.:40421-52-1
- Capsaicin
Catalog No.:BCN1016
CAS No.:404-86-4
- Pamidronate
Catalog No.:BCC4693
CAS No.:40391-99-9
- 6-Methoxykaempferol 3-O-rutinoside
Catalog No.:BCN2379
CAS No.:403861-33-6
- Benzyl 2-hydroxy-6-(beta-glucosyloxy)benzoate
Catalog No.:BCN7434
CAS No.:403857-21-6
- HEMADO
Catalog No.:BCC7118
CAS No.:403842-38-6
- Z-Lys(Z)-OH
Catalog No.:BCC2762
CAS No.:405-39-0
- 4'-Demethylpodophyllotoxin
Catalog No.:BCN2625
CAS No.:40505-27-9
- Salubrinal
Catalog No.:BCC4843
CAS No.:405060-95-9
- Drechslerine A
Catalog No.:BCN7561
CAS No.:405157-84-8
- Drechslerine D
Catalog No.:BCN7502
CAS No.:405157-88-2
- Besifloxacin HCl
Catalog No.:BCC4764
CAS No.:405165-61-9
- CHIR-124
Catalog No.:BCC3750
CAS No.:405168-58-3
- Dovitinib (TKI-258, CHIR-258)
Catalog No.:BCC1169
CAS No.:405169-16-6
- NFPS
Catalog No.:BCC7484
CAS No.:405225-21-0
- Dadahol A
Catalog No.:BCN5457
CAS No.:405281-76-7
- Cyclapolin 9
Catalog No.:BCC7571
CAS No.:40533-25-3
- SB590885
Catalog No.:BCC4392
CAS No.:405554-55-4
Optimization of the in vitro cardiac safety of hydroxamate-based histone deacetylase inhibitors.[Pubmed:21650221]
J Med Chem. 2011 Jul 14;54(13):4752-72.
Histone deacetylase (HDAC) inhibitors have shown promise in treating various forms of cancer. However, many HDAC inhibitors from diverse structural classes have been associated with QT prolongation in humans. Inhibition of the human ether a-go-go related gene (hERG) channel has been associated with QT prolongation and fatal arrhythmias. To determine if the observed cardiac effects of HDAC inhibitors in humans is due to hERG blockade, a highly potent HDAC inhibitor devoid of hERG activity was required. Starting with dacinostat (LAQ824), a highly potent HDAC inhibitor, we explored the SAR to determine the pharmacophores required for HDAC and hERG inhibition. We disclose here the results of these efforts where a high degree of pharmacophore homology between these two targets was discovered. This similarity prevented traditional strategies for mitigating hERG binding/modulation from being successful and novel approaches for reducing hERG inhibition were required. Using a hERG homology model, two compounds, 11r and 25i, were discovered to be highly efficacious with weak affinity for the hERG and other ion channels.
Conformational refinement of hydroxamate-based histone deacetylase inhibitors and exploration of 3-piperidin-3-ylindole analogues of dacinostat (LAQ824).[Pubmed:20205394]
J Med Chem. 2010 Apr 8;53(7):2952-63.
Inspired by natural product HDAC inhibitors, we prepared a series of conformationally restrained HDAC inhibitors based on the hydroxamic acid dacinostat (LAQ824, 7). Several scaffolds with improved biochemical and cellular potency, as well as attenuated hERG inhibition, were identified, suggesting that the introduction of molecular rigidity is a viable strategy to enhance HDAC binding and mitigate hERG liability. Further SAR studies around a 3-piperidin-3-ylindole moiety resulted in the discovery of compound 30, for which a unique conformation was speculated to contribute to overcoming increased lipophilicity and attenuating hERG binding. Separation of racemate 30 afforded 32, the R enantiomer, which demonstrated improved potency in both enzyme and cellular assays compared to dacinostat.
Targeting Histone Deacetylases in Malignant Melanoma: A Future Therapeutic Agent or Just Great Expectations?[Pubmed:28982843]
Anticancer Res. 2017 Oct;37(10):5355-5362.
BACKGROUND/AIM: Malignant melanoma is the most aggressive type of skin cancer, with increasing frequency and mortality. Melanoma is characterized by rapid proliferation and metastases. Malignant transformation of normal melanocytes is associated with imbalance between oncogenes' action and tumor suppressor genes. Mutations or inactivation of these genes plays an important role in the pathogenesis of malignant melanoma. Many target-specific agents improved progression-free survival but unfortunately metastatic melanoma remains incurable, so new therapeutic strategies are needed. The balance of histones' acetylation affects cell cycle progression, differentiation and apoptosis. Histone deacetylases (HDAC) are associated with different types of cancer. Histone deacetylase inhibitors (HDACI) are enzymes that inhibit the action of HDAC, resulting in block of tumor cell proliferation. A small number of these enzymes has been studied regarding their anticancer effects in melanoma. The purpose of this article was to review the therapeutic effect of HDACI against malignant melanoma, enlightening the molecular mechanisms of their action. MATERIALS AND METHODS: The MEDLINE database was used. The keywords/ phrases were; HDACI, melanoma, targeted therapies for melanoma. Our final conclusions were based on studies that didn't refer solely to melanoma due to their wider experimental data. Thirty-two articles were selected from the total number of the search's results. Only English articles published until March 2017 were used. RESULTS: Molecules, such as valproid acid (VPA), LBH589, LAQ824 (dacinostat), vorinostat, tubacin, sirtinol and tx-527, suberoyl bis-hydroxamic acid (SBHA), depsipeptide and Trichostatin A (TSA) have shown promising antineoplastic effects against melanoma. CONCLUSION: HDACI represent a promising agent for targeted therapy. More trials are required.


