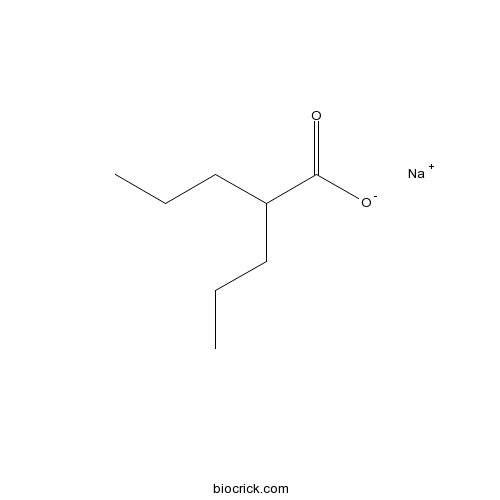
Valproic acid sodium salt (Sodium valproate)HDAC inhibitor CAS# 1069-66-5 |
- Resminostat hydrochloride
Catalog No.:BCC1888
CAS No.:1187075-34-8
- RG2833
Catalog No.:BCC1893
CAS No.:1215493-56-3
- Daminozide
Catalog No.:BCC1514
CAS No.:1596-84-5
- Tasquinimod
Catalog No.:BCC1987
CAS No.:254964-60-8
- CHAPS
Catalog No.:BCC1476
CAS No.:75621-03-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1069-66-5 | SDF | Download SDF |
| PubChem ID | 16760703 | Appearance | Powder |
| Formula | C8H15NaO2 | M.Wt | 166.19 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | VPA, Sodium Valproate | ||
| Solubility | H2O : ≥ 48 mg/mL (288.83 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | sodium;2-propylpentanoate | ||
| SMILES | CCCC(CCC)C(=O)[O-].[Na+] | ||
| Standard InChIKey | AEQFSUDEHCCHBT-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C8H16O2.Na/c1-3-5-7(6-4-2)8(9)10;/h7H,3-6H2,1-2H3,(H,9,10);/q;+1/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Histone deacetylase inhibitor (IC50 = 400 μM) that exhibits anticancer, anti-inflammatory and neuroprotective effects. Displays anticonvulsive activity via an increase in GABA levels and decreases Aβ production in animal models of Alzheimer's disease. Also attenuates NMDA-mediated excitation, blocks voltage-gated Na+ channels and modulates firing of neurons. Enables induction of pluripotent stem cells from somatic cells by Oct4 and Sox2. Can induce autophagy by inhibiting inositol synthesis. |

Valproic acid sodium salt (Sodium valproate) Dilution Calculator

Valproic acid sodium salt (Sodium valproate) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.0172 mL | 30.086 mL | 60.1721 mL | 120.3442 mL | 150.4302 mL |
| 5 mM | 1.2034 mL | 6.0172 mL | 12.0344 mL | 24.0688 mL | 30.086 mL |
| 10 mM | 0.6017 mL | 3.0086 mL | 6.0172 mL | 12.0344 mL | 15.043 mL |
| 50 mM | 0.1203 mL | 0.6017 mL | 1.2034 mL | 2.4069 mL | 3.0086 mL |
| 100 mM | 0.0602 mL | 0.3009 mL | 0.6017 mL | 1.2034 mL | 1.5043 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 0.4 mM
Valproic acid (VPA) is a histone deacetylase inhibitor.
Histone deacetylases (HDACs), which have been recognized for their roles in controlling chromatin remodeling through histone acetylation/deacetylation, are currently known to modify a large number of non-histone proteins to regulate diverse cell processes.
In vitro: VPA showed to have cellular neuroprotective properties. In cultured neurons, VPA protected from thapsigargin-induced endoplasmic reticulum stress, glutamate-induced excitotoxicity, as well as lipopolysaccharide (LPS)-induced dopaminergic neuronal death. In midbrain neuron-glia cultures, VPA was also shown to inhibit LPS-induced, microglia-mediated inflammation [1].
In vivo: Post-pMCAO injections with VPA could decrease the brain infarct volume. Postinsult treatment with VPA also reduced the number of microglia, suppressed microglial activation, and inhibited other inflammatory markers in the ischemic brain. The reduction in acetylated histone H3 was prevented by treatment with VPA. Moreover, VPA superinduced heat-shock protein 70 and blocked pMCAO-induced down-regulation of cyclooxygenase-2. The sensory, motor, and reflex performance of pMCAO rats was improved by VPA treatment [1].
Clinical trial: In CRPC patients, VPA levels and VPA exposure duration were independent predictors for declining prostate-specific antigen (PSA). Oral VPA was not well tolerated in this patient population due to grade 1 and 2 neurologic symptoms and fatigue. Total and free VPA levels were useful for preventing severe toxicities from oral VPA. It was unlikely that PSA responses from oral VPA were related to HDAC inhibition [2].
References:
[1] Kim HJ,Rowe M,Ren M,Hong JS,Chen PS,Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther.2007 Jun;321(3):892-901. Epub 2007 Mar 19.
[2] Sharma S,Symanowski J,Wong B,Dino P,Manno P,Vogelzang N. A Phase II Clinical Trial of Oral Valproic Acid in Patients with Castration-Resistant Prostate Cancers Using an Intensive Biomarker Sampling Strategy. Transl Oncol.2008 Sep;1(3):141-7.
- 5-Allyl-3-methoxy-6-methyl-7-(3,4,5-trimethoxyphenyl)bicyclo[3.2.1]oct-3-ene-2,8-dione
Catalog No.:BCN1632
CAS No.:106894-43-3
- BIBP 3226 trifluoroacetate
Catalog No.:BCC7456
CAS No.:1068148-47-9
- Diethyl Acetamidomalonate
Catalog No.:BCC2841
CAS No.:1068-90-2
- Otophylloside B
Catalog No.:BCN7267
CAS No.:106758-54-7
- 24-Deacetylalisol O
Catalog No.:BCN3365
CAS No.:1067510-31-9
- GYY 4137 morpholine salt
Catalog No.:BCC7739
CAS No.:106740-09-4
- Olprinone
Catalog No.:BCC1820
CAS No.:106730-54-5
- Boc-D-Lys-OH
Catalog No.:BCC3420
CAS No.:106719-44-2
- Matairesinol 4'-O-beta-gentiobioside
Catalog No.:BCN2848
CAS No.:106647-14-7
- Trachelosiaside
Catalog No.:BCN7743
CAS No.:106647-12-5
- Qingyangshengenin A
Catalog No.:BCN8126
CAS No.:106644-33-1
- 3,10-Dihydroxy-5,11-dielmenthadiene-4,9-dione
Catalog No.:BCN1633
CAS No.:106623-23-8
- Boc-D-Leucinol
Catalog No.:BCC2723
CAS No.:106930-51-2
- Adefovir
Catalog No.:BCC8808
CAS No.:106941-25-7
- Boc-isoleucinol
Catalog No.:BCC3096
CAS No.:106946-74-1
- Propylamine
Catalog No.:BCN1814
CAS No.:107-10-8
- Taurine
Catalog No.:BCN1750
CAS No.:107-35-7
- Betaine
Catalog No.:BCN6303
CAS No.:107-43-7
- N-Methyltaurine
Catalog No.:BCN1751
CAS No.:107-68-6
- 3-Methyl-1-butylamine
Catalog No.:BCN1810
CAS No.:107-85-7
- H-ß-Ala-OH
Catalog No.:BCC2851
CAS No.:107-95-9
- Sarcosine
Catalog No.:BCN2744
CAS No.:107-97-1
- Granisetron HCl
Catalog No.:BCC1060
CAS No.:107007-99-8
- Amyloid Beta-Peptide (12-28) (human)
Catalog No.:BCC1044
CAS No.:107015-83-8
Infusion of Valproic Acid Into the Renal Medulla Activates Stem Cell Population and Attenuates Salt-Sensitive Hypertension in Dahl S Rats.[Pubmed:28693025]
Cell Physiol Biochem. 2017;42(3):1264-1273.
BACKGROUND: Our previous study has detected a stem cell deficiency in the renal medulla in Dahl salt-sensitive (S) rats. This study determined whether infusion of valproic acid (VA), an agent known to stimulate the stem cell function, attenuated salt-sensitive hypertension in Dahl S rats. METHODS: Uninephrectomized Dahl S rats were infused with vehicle or VA (50mg/kg/d) into the renal medulla and fed with a low (LS) or high salt diet (HS). Stem cell marker and number were analyzed by immunohistochemistry, Real-time RT-PCR and Western blot. Sodium excretion and blood pressure were measured. RESULTS: VA significantly increased the mRNA and protein levels of FGF2, a stem cell niche factor, and CD133, a stem cell marker. The number of CD133+ cells was significantly increased in the renal medulla in VA-treated rats. Meanwhile, high salt-induced increases in the mRNA level of proinflammatory factors interleukin-1beta and interleukin-6 were blocked in VA-treated rats. Functionally, sodium excretion in response to the blood pressure increase and acute sodium loading was significantly enhanced, sodium retention attenuated, high salt-induced increase of blood pressure reduced in VA-treated rats. CONCLUSION: Activation of stem cell function by VA inhibits the activation of proinflammatory factors and attenuates salt-sensitive hypertension in Dahl S rats.
A combination of valproic acid sodium salt, CHIR99021, E-616452, tranylcypromine, and 3-Deazaneplanocin A causes stem cell-like characteristics in cancer cells.[Pubmed:28881812]
Oncotarget. 2017 Jun 7;8(32):53302-53312.
Many studies are based on the hypothesis that recurrence and drug resistance in lung carcinoma are due to a subpopulation of cancer stem-like cells (CSLCs) in solid tumors. Therefore it is crucial to screen for and recognize lung CSLCs. In this study, we stimulated non-small cell lung cancer (NSCLC) A549 cells to display stem cell-like characteristics using a combination of five small molecule compounds. The putative A549 stem cells activated an important CSLC marker, CD133 protein, as well multiple CSLC-related genes including ATP-binding cassette transporter G2 (ABCG2), C-X-C chemokine receptor type 4 (CXCR4), NESTIN, and BMI1. The A549 stem-like cells displayed resistance to the chemotherapeutic drugs etoposide and cisplatin, epithelial-to-mesenchymal transition properties, and increased protein expression levels of NOTCH1 and Hes Family bHLH Transcription Factor 1 (HES1). When A549 cells were pretreated with a NOTCH signaling pathway inhibitor before compound induction, expression of the NOTCH1 target gene HES1 was reduced. This demonstrated that the NOTCH signaling pathway in the putative A549 stem-like cells had been activated. Together, the results of our study showed that a combination of five small molecule agents could transform A549 cells into putative stem-like cells, and that these compounds could also elevate CD133 and ABCG2 protein expression levels in H460 cells. This study provides a convenient method for obtaining lung CSLCs, which may be an effective strategy for developing lung carcinoma treatments.
YM155 induces apoptosis through proteasome-dependent degradation of MCL-1 in primary effusion lymphoma.[Pubmed:28396094]
Pharmacol Res. 2017 Jun;120:242-251.
Primary effusion lymphoma (PEL) is a lymphoma that shows malignant effusion in body cavities without contiguous tumor masses and has a very poor prognosis. We recently developed a novel drug screening system using patient-derived xenograft (PDX) cells that maintained the primary cell phenotype better than cell lines. This screening is expected to discover anti-tumor drugs that have been overlooked by conventional screening using cell lines. We herein performed this screening to identify new therapeutic agents for PEL. We screened 3518 compounds with known pharmaceutical activities based on cytotoxic effects on PDX cells of PEL and selected YM155, a possible survivin inhibitor. It exerted strong anti-tumor effects in PDX cells and three cell lines of PEL; the GI50 of YM155 was 1.2-7.9nM. We found that YM155 reduced myeloid cell leukemia-1 (MCL-1) protein levels prior to decreasing survivin levels, and this was inhibited by a proteasome inhibitor. The knockdown of MCL-1 by siRNA induced cell death in a PEL cell line, suggesting the involvement of decreased MCL-1 levels in YM155-induced cell death. YM155 also induced the phosphorylation of ERK1/2 and MCL-1, and a MEK1 inhibitor inhibited the phosphorylation of ERK1/2, degradation of MCL-1, and YM155-induced apoptosis. These results indicate that YM155 induces the proteasome-dependent degradation of MCL-1 through its phosphorylation by ERK1/2 and causes apoptosis in PEL cells. Furthermore, a treatment with YM155 significantly inhibited the development of ascites in PEL PDX mice. These results suggest the potential of YM155 as an anti-cancer agent for PEL.
Mechanisms for Improved Hygroscopicity of L-Arginine Valproate Revealed by X-Ray Single Crystal Structure Analysis.[Pubmed:27986291]
J Pharm Sci. 2017 Mar;106(3):859-865.
Valproic acid is widely used as an antiepileptic agent. Valproic acid is in liquid phase while sodium valproate is in solid phase at room temperature. Sodium valproate is hard to manufacture because of its hygroscopic and deliquescent properties. To improve these, cocrystal and salt screening for valproic acid was employed in this study. Two solid salt forms, l-arginine valproate and l-lysine valproate, were obtained and characterized. By using dynamic vapor sorption method, the critical relative humidity of sodium valproate, l-arginine valproate, and l-lysine valproate were measured. Critical relative humidity of sodium valproate was 40%, of l-lysine valproate was 60%, and of l-arginine valproate was 70%. Single-crystal X-ray structure determination of l-arginine valproate was employed. l-Lysine valproate was of low diffraction quality, and l-arginine valproate formed a 1:1 salt. Crystal l-arginine valproate has a disorder in the methylene carbon chain that creates 2 conformations. The carboxylate group of valproic acid is connected to the amino group of l-arginine. Crystalline morphologies were calculated from its crystal structure. Adsorption of water molecules to crystal facets was simulated by Material Studio. When comparing adsorption energy per site of these salts, sodium valproate is more capable of adsorption of water molecule than l-arginine valproate.
Docking and QSAR Studies of Aryl-valproic Acid Derivatives to Identify Antiproliferative Agents Targeting the HDAC8.[Pubmed:27774878]
Anticancer Agents Med Chem. 2017;17(7):927-940.
BACKGROUND: Histone deacetylase 8 (HDAC8) is a plausible target for the development of novel anticancer drugs using a metal-chelating group and hydrophobic moieties as pharmacophores. It is known that valproic acid (administered as its salt, sodium valproate; VPANa+) is an HDAC8 inhibitor characterized by its hydrophobic chains. Nevertheless, VPA is hepatotoxic and VPA analogues might be explored for less hepatotoxic antiproliferative compounds. METHOD: In this work, docking and QSAR studies of 500 aryl-VPA derivatives as possible HDAC8 inhibitors were performed in order to explore and select potential anti-proliferative compounds. Docking results identified pi-pi, hydrogen bonds as the most important noncovalent interactions between HDAC8 (PDB: 3F07) and the ligands tested, whereas Belm4 was the best QSAR descriptor and classified as a 2D-BCUT descriptor. RESULT: Based on theoretical studies, compound DAVP042 was synthesized and evaluated in vitro for its antiproliferative activities on several cancer cell lines (A549-lung, MCF-7-breast, HCT116-colon and U937- lymphoid tissue) in comparison to VPA, as well as for its inhibitory activity on HDAC8 using in vitro models. DAVP042 demonstrated to have antiproliferative activity on all cancer cell lines employed, not only suggesting that this compound should be further studied, but also demonstrating that the methodology herein employed is appropriated to identify new therapeutic candidates.
Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2.[Pubmed:18849973]
Nat Biotechnol. 2008 Nov;26(11):1269-75.
Ectopic expression of defined sets of genetic factors can reprogram somatic cells to induced pluripotent stem (iPS) cells that closely resemble embryonic stem (ES) cells. The low efficiency with which iPS cells are derived hinders studies on the molecular mechanism of reprogramming, and integration of viral transgenes, in particular the oncogenes c-Myc and Klf4, may handicap this method for human therapeutic applications. Here we report that valproic acid (VPA), a histone deacetylase inhibitor, enables reprogramming of primary human fibroblasts with only two factors, Oct4 and Sox2, without the need for the oncogenes c-Myc or Klf4. The two factor-induced human iPS cells resemble human ES cells in pluripotency, global gene expression profiles and epigenetic states. These results support the possibility of reprogramming through purely chemical means, which would make therapeutic use of reprogrammed cells safer and more practical.
Valproic acid inhibits Abeta production, neuritic plaque formation, and behavioral deficits in Alzheimer's disease mouse models.[Pubmed:18955571]
J Exp Med. 2008 Nov 24;205(12):2781-9.
Neuritic plaques in the brains are one of the pathological hallmarks of Alzheimer's disease (AD). Amyloid beta-protein (Abeta), the central component of neuritic plaques, is derived from beta-amyloid precursor protein (APP) after beta- and gamma-secretase cleavage. The molecular mechanism underlying the pathogenesis of AD is not yet well defined, and there has been no effective treatment for AD. Valproic acid (VPA) is one of the most widely used anticonvulsant and mood-stabilizing agents for treating epilepsy and bipolar disorder. We found that VPA decreased Abeta production by inhibiting GSK-3beta-mediated gamma-secretase cleavage of APP both in vitro and in vivo. VPA treatment significantly reduced neuritic plaque formation and improved memory deficits in transgenic AD model mice. We also found that early application of VPA was important for alleviating memory deficits of AD model mice. Our study suggests that VPA may be beneficial in the prevention and treatment of AD.
Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action.[Pubmed:17371805]
J Pharmacol Exp Ther. 2007 Jun;321(3):892-901.
The pathophysiology of cerebral ischemia involves multiple mechanisms including neuroinflammation mediated by activated microglia and infiltrating macrophages/monocytes. The present study employed a rat permanent middle cerebral artery occlusion (pMCAO) model to study effects of histone deacetylase (HDAC) inhibition on ischemia-induced brain infarction, neuroinflammation, gene expression, and neurological deficits. We found that post-pMCAO injections with HDAC inhibitors, valproic acid (VPA), sodium butyrate (SB), or trichostatin A (TSA), decreased brain infarct volume. Postinsult treatment with VPA or SB also suppressed microglial activation, reduced the number of microglia, and inhibited other inflammatory markers in the ischemic brain. The reduction in levels of acetylated histone H3 in the ischemic brain was prevented by treatment with VPA, SB, or TSA. Moreover, injections with HDAC inhibitors superinduced heat-shock protein 70 and blocked pMCAO-induced down-regulation of phospho-Akt, as well as ischemia-elicited up-regulation of p53, inducible nitric oxide synthase, and cyclooxygenase-2. The motor, sensory, and reflex performance of pMCAO rats was improved by VPA, SB, or TSA treatment. The beneficial effects of SB and VPA in reducing brain infarct volume and neurological deficits occurred when either drug was administrated at least 3 h after ischemic onset, and the behavioral improvement was long-lasting. Together, our results demonstrate robust neuroprotective effects of HDAC inhibitors against cerebral ischemia-induced brain injury. The neuroprotection probably involves multiple mechanisms including suppression of ischemia-induced cerebral inflammation. Given that there is no effective treatment for stroke, HDAC inhibitors, such as VPA, SB, and TSA, should be evaluated for their potential use for clinical trials in stroke patients.
Valproic acid, a molecular lead to multiple regulatory pathways.[Pubmed:17448293]
Folia Biol (Praha). 2007;53(2):37-49.
Valproic acid (2-propyl pentanoic acid) is a drug used for the treatment of epilepsy and bipolar disorder. Although very rare, side effects such as spina bifida and other defects of neural tube closure indicate that valproic acid interferes with developmental regulatory pathways. Recently obtained data show that valproic acid affects cell growth, differentiation, apoptosis and immunogenicity of cultured cancer cells and tumours. Focused studies uncovered the potential of valproic acid to interfere with multiple regulatory mechanisms including histone deacetylases, GSK3 alpha and beta, Akt, the ERK pathway, the phosphoinositol pathway, the tricarboxylic acid cycle, GABA, and the OXPHOS system. Valproic acid is emerging as a potential anticancer drug and may also serve as a molecular lead that can help design drugs with more specific and more potent effects on the one side and drugs with wide additive but weaker effects on the other. Valproic acid is thus a powerful molecular tool for better understanding and therapeutic targeting of pathways that regulate the behaviour of cancer cells.
Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen.[Pubmed:11473107]
J Biol Chem. 2001 Sep 28;276(39):36734-41.
Valproic acid is widely used to treat epilepsy and bipolar disorder and is also a potent teratogen, but its mechanisms of action in any of these settings are unknown. We report that valproic acid activates Wntdependent gene expression, similar to lithium, the mainstay of therapy for bipolar disorder. Valproic acid, however, acts through a distinct pathway that involves direct inhibition of histone deacetylase (IC(50) for HDAC1 = 0.4 mm). At therapeutic levels, valproic acid mimics the histone deacetylase inhibitor trichostatin A, causing hyperacetylation of histones in cultured cells. Valproic acid, like trichostatin A, also activates transcription from diverse exogenous and endogenous promoters. Furthermore, valproic acid and trichostatin A have remarkably similar teratogenic effects in vertebrate embryos, while non-teratogenic analogues of valproic acid do not inhibit histone deacetylase and do not activate transcription. Based on these observations, we propose that inhibition of histone deacetylase provides a mechanism for valproic acid-induced birth defects and could also explain the efficacy of valproic acid in the treatment of bipolar disorder.


