CHAPSZwitterionic detergent for membrane proteins,nondenaturing CAS# 75621-03-3 |
- Resminostat hydrochloride
Catalog No.:BCC1888
CAS No.:1187075-34-8
- RG2833
Catalog No.:BCC1893
CAS No.:1215493-56-3
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- Daminozide
Catalog No.:BCC1514
CAS No.:1596-84-5
- Tasquinimod
Catalog No.:BCC1987
CAS No.:254964-60-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 75621-03-3 | SDF | Download SDF |
| PubChem ID | 107670 | Appearance | Powder |
| Formula | C32H58N2O7S | M.Wt | 614.88 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 46 mg/mL (74.81 mM) *"≥" means soluble, but saturation unknown. | ||
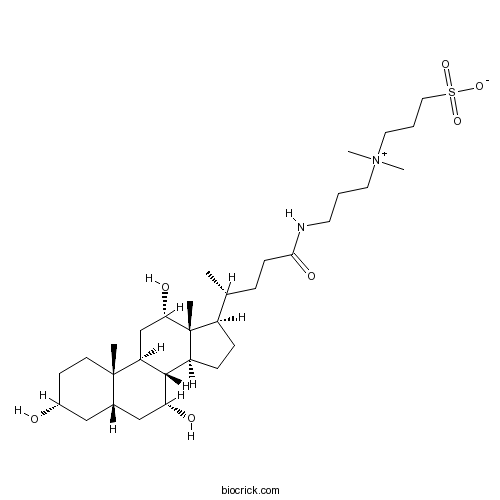
| Chemical Name | 3-[dimethyl-[3-[[(4R)-4-[(3R,5S,7R,8R,9S,10S,12S,13R,14S,17R)-3,7,12-trihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoyl]amino]propyl]azaniumyl]propane-1-sulfonate | ||
| SMILES | CC(CCC(=O)NCCC[N+](C)(C)CCCS(=O)(=O)[O-])C1CCC2C1(C(CC3C2C(CC4C3(CCC(C4)O)C)O)O)C | ||
| Standard InChIKey | UMCMPZBLKLEWAF-BCTGSCMUSA-N | ||
| Standard InChI | InChI=1S/C32H58N2O7S/c1-21(8-11-29(38)33-14-6-15-34(4,5)16-7-17-42(39,40)41)24-9-10-25-30-26(20-28(37)32(24,25)3)31(2)13-12-23(35)18-22(31)19-27(30)36/h21-28,30,35-37H,6-20H2,1-5H3,(H-,33,38,39,40,41)/t21-,22+,23-,24-,25+,26+,27-,28+,30+,31+,32-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CHAPS is a zwitterionic nondenaturing detergent for solubilizing membrane proteins. |

CHAPS Dilution Calculator

CHAPS Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6263 mL | 8.1317 mL | 16.2633 mL | 32.5267 mL | 40.6583 mL |
| 5 mM | 0.3253 mL | 1.6263 mL | 3.2527 mL | 6.5053 mL | 8.1317 mL |
| 10 mM | 0.1626 mL | 0.8132 mL | 1.6263 mL | 3.2527 mL | 4.0658 mL |
| 50 mM | 0.0325 mL | 0.1626 mL | 0.3253 mL | 0.6505 mL | 0.8132 mL |
| 100 mM | 0.0163 mL | 0.0813 mL | 0.1626 mL | 0.3253 mL | 0.4066 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CHAPS is a zwitterionic nondenaturing detergent for solubilizing membrane proteins. CHAPS is often used as a detergent in the solubilization and purification of membrane proteins for several advantageous reasons. CHAPS detergent is non-denaturing to membrane proteins, can solubilize proteins, disaggregate protein-protein interactions and is electrically neutral. CHAPS is also useful in ion exchange chromatography and isoelectric focusing as it is zwitterionic and does not exhibit a net charge between pH 2 to 12. The critical micelle concentration of CHAPS is 6-10mM.
- (-)-Usnic acid
Catalog No.:BCN4306
CAS No.:7562-61-0
- (S)-(+)-α-Methylhistamine dihydrobromide
Catalog No.:BCC6700
CAS No.:75614-93-6
- Fludarabine Phosphate (Fludara)
Catalog No.:BCC3681
CAS No.:75607-67-9
- Methyl 3,4,5-trimethoxycinnamate
Catalog No.:BCN4589
CAS No.:7560-49-8
- Kaerophyllin
Catalog No.:BCN4304
CAS No.:75590-33-9
- alpha-Tocopherolquinone
Catalog No.:BCN4305
CAS No.:7559-04-8
- Sodium phosphate monobasic
Catalog No.:BCC8033
CAS No.:7558-80-7
- Sodium phosphate dibasic
Catalog No.:BCC7585
CAS No.:7558-79-4
- 20-Deoxyingenol 3-angelate
Catalog No.:BCN6642
CAS No.:75567-38-3
- Ingenol 3-Angelate
Catalog No.:BCN2961
CAS No.:75567-37-2
- Dencichin
Catalog No.:BCN2555
CAS No.:7554-90-7
- Moxonidine hydrochloride
Catalog No.:BCC5163
CAS No.:75536-04-8
- Moracenin B
Catalog No.:BCC8341
CAS No.:75629-19-5
- Gomisin K1
Catalog No.:BCN7030
CAS No.:75629-20-8
- Oncrasin 1
Catalog No.:BCC2390
CAS No.:75629-57-1
- Knightolamine
Catalog No.:BCN1912
CAS No.:75638-70-9
- Chalcostrobamine
Catalog No.:BCN1900
CAS No.:75638-72-1
- DSLET
Catalog No.:BCC5718
CAS No.:75644-90-5
- Boc-D-Asn-OH
Catalog No.:BCC3362
CAS No.:75647-01-7
- Strobamine
Catalog No.:BCN1943
CAS No.:75656-91-6
- 4-Acetyl-1,1-dimethylpiperazinium iodide
Catalog No.:BCC6616
CAS No.:75667-84-4
- 2,4-Dihydroxy-4,6-dimethoxydihydrochalcone
Catalog No.:BCN1363
CAS No.:75679-58-2
- Isradipine (Dynacirc)
Catalog No.:BCC3797
CAS No.:75695-93-1
- 5-Amino-2-methylindole
Catalog No.:BCC8731
CAS No.:7570-49-2
Obtaining Soluble Folded Proteins from Inclusion Bodies Using Sarkosyl, Triton X-100, and CHAPS: Application to LB and M9 Minimal Media.[Pubmed:27038270]
Curr Protoc Protein Sci. 2016 Apr 1;84:6.13.1-6.13.24.
This unit describes a straightforward and efficient method of using sarkosyl to solubilize and recover difficult recombinant proteins, such as GST- and His6 -tagged fusion proteins, that are overexpressed in E. coli. This protocol is especially useful for rescuing recombinant proteins overexpressed in M9 minimal medium. Sarkosyl added to lysis buffers helps with both protein solubility and cell lysis. Higher percentage sarkosyl (up to 10%) can extract >95% of soluble protein from inclusion bodies. In the case of sarkosyl-solubilized GST-fusion proteins, batch-mode affinity purification requires addition of a specific ratio of Triton X-100 and CHAPS, while sarkosyl-solubilized His6 -tagged fusion proteins can be directly purified on Ni(2+) resin columns. Proteins purified by this method could be widely used in biological assays, structure analysis and mass spectrum assay.
Study protocol of "CHAPS": a randomized controlled trial protocol of Care Coordination for Health Promotion and Activities in Parkinson's Disease to improve the quality of care for individuals with Parkinson's disease.[Pubmed:26670300]
BMC Neurol. 2015 Dec 15;15:258.
BACKGROUND: Parkinson's disease, the second most common neurodegenerative disease, is diagnostically defined by motor impairments, but also includes often under-recognized impairments in cognition, mood, sleep, and the autonomic nervous system. These problems can severely affect individuals' quality of life. In our prior research, we have developed indicators to measure the quality of care delivered to patients with Parkinson's disease, and we identified gaps in delivering evidence-based treatments for this population. Effective strategies to close these gaps are needed to improve patient quality of life. METHODS/DESIGN: Building on prior research we developed a multi-faceted proactive implementation program called Care Coordination for Health Promotion and Activities in Parkinson's Disease (CHAPS). To be eligible, patients had to have at least two visits with a primary diagnosis of idiopathic Parkinson's disease (ICD-9 code: 332.0) at one of five Veterans Affairs Medical Centers in the southwestern United States from 2010 to 2014. The program consists of telephone assessments, evidence-based protocols, and tools to enhance patient self-management, care planning, and coordination of care across providers, including an electronic database to support and track coordination of care. Our mixed-methods study employs a randomized, controlled trial design to test whether the CHAPS intervention improves performance in 38 quality measures among an analytic sample of 346 patients. The 38 quality measures are categorized into overarching areas of communication, education, and continuity; regulatory reporting; diagnosis; periodic assessment; medication use; management of motor and non-motor symptoms; use of non-pharmacological approaches and therapies; palliative care; and health maintenance. Secondary outcomes are patient health-related quality of life, self-efficacy, and perceptions of care quality. We are also evaluating the extent of the CHAPS Program implementation and measuring program costs and impacts on health services utilization, in order to perform a analysis of the CHAPS program from the perspective of the Veterans Health Administration (VA). Outcomes are assessed by interviewer-administered surveys collected at baseline and at 6, 12, and 18 months, and by medical record chart abstractions. Analyses will be intention-to-treat. DISCUSSION: The CHAPS Program is poised for dissemination within the VA National Parkinson's Disease Research, Education, and Clinical Center Consortium if demonstrated efficacious. TRIAL REGISTRATION: ClinicalTrials.gov NCT01532986; registered on January 13, 2012.
Impact of purification conditions and history on A2A adenosine receptor activity: The role of CHAPS and lipids.[Pubmed:27241126]
Protein Expr Purif. 2016 Aug;124:62-7.
The adenosine A2A receptor (A2AR) is a much-studied class A G protein-coupled receptor (GPCR). For biophysical studies, A2AR is commonly purified in a detergent mixture of dodecylmaltoside (DDM), 3-(3-cholamidopropyl) dimethylammoniopropane sulfonate (CHAPS), and cholesteryl hemisuccinate (CHS). Here we studied the effects of CHAPS on the ligand binding activity and stability of wild type, full-length human A2AR. We also tested the cholesterol requirement for maintaining the active conformation of the receptor when solubilized in detergent micelles. To this end, the receptor was purified using DDM, DDM/CHAPS, or the short hydrocarbon chain lipid 1,2-dihexanoyl-sn-glycero-3-phosphocholine (DHPC, di-6:0PC). After solubilization in DDM, DDM/CHAPS, or DHPC micelles, although A2AR was found to retain its native-like fold, its binding ability was significantly compromised compared to DDM or DDM/CHAPS with CHS. It therefore appears that although cholesterol is not needed for A2AR to retain a native-like, alpha-helical conformation, it may be a critical component for high affinity ligand binding. Further, this result suggests that the conformational differences between the active and inactive protein may be so subtle that commonly used spectroscopic methods are unable to differentiate between the two forms, highlighting the need for activity measurements. The studies presented in this paper also underline the importance of the protein's purification history; i.e., detergents that interact with the protein during purification affect the ligand binding properties of the receptor in an irreversible manner.
Blocking the interaction between S100A9 and RAGE V domain using CHAPS molecule: A novel route to drug development against cell proliferation.[Pubmed:27524699]
Biochim Biophys Acta. 2016 Nov;1864(11):1558-69.
Human S100A9 (Calgranulin B) is a Ca(2+)-binding protein, from the S100 family, that often presents as a homodimer in myeloid cells. It becomes an important mediator during inflammation once calcium binds to its EF-hand motifs. Human RAGE protein (receptor for advanced glycation end products) is one of the target-proteins. RAGE binds to a hydrophobic surface on S100A9. Interactions between these proteins trigger signal transduction cascades, promoting cell growth, proliferation, and tumorigenesis. Here, we present the solution structure of mutant S100A9 (C3S) homodimer, determined by multi-dimensional NMR experiments. We further characterize the solution interactions between mS100A9 and the RAGE V domain via NMR spectroscopy. CHAPS is a zwitterionic and non-denaturing molecule widely used for protein solubilizing and stabilization. We found out that CHAPS and RAGE V domain would interact with mS100A9 by using (1)H-(15)N HSQC NMR titrations. Therefore, using the HADDOCK program, we superimpose two binary complex models mS100A9-RAGE V domain and mS100A9-CHAPS and demonstrate that CHAPS molecules could play a crucial role in blocking the interaction between mS100A9 and the RAGE V domain. WST-1 assay results also support the conclusion that CHAPS inhibits the bioactivity of mS100A9. This report will help to inform new drug development against cell proliferation.
Preparative two-dimensional gel electrophoresis of membrane proteins.[Pubmed:9527487]
Electrophoresis. 1997 Dec;18(14):2573-81.
Electrophoretic techniques, and especially two-dimensional gel electrophoresis (2-DE), have provided an indispensable set of tools for the separation of complex protein mixtures as well as for the identification of protein-protein interactions. Nevertheless, after its introduction more than twenty years ago and even with recent technical developments, the separation of integral and peripheral membrane proteins, in amounts sufficient for microsequencing, is still a difficult task. Lipids present in the membrane as well as the low solubility of hydrophobic membrane proteins result in protein aggregation both on the sample application point and on isoelectric focusing. As a consequence many proteins do not enter the first or second dimension of 2-DE. Here we describe the modification of a protocol using a combination of 3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate (CHAPS), chaotropic agents (thiourea, urea), Tris base and reducing agents (1,4-dithioerythritol) to improve solubilization of integral and peripheral membrane proteins. Preparative amounts of membrane proteins (up to 2 mg) were loaded during reswelling of dry immobilized pH gradients and the resulting Coomassie staining patterns were largely superimposable with silver-stained gels obtained from identical samples (4 microg). This indicates that the recovery of proteins from the sample is not significantly compromised by the scale-up procedure. A direct application of this method for the characterization and identification of membrane proteins from cellular organelles is described in another paper in this issue (I. Fialka et al., Electrophoresis 1997, 18, 2582-2590).
Differential solubilization of lipids along with membrane proteins by different classes of detergents.[Pubmed:7586093]
Chem Phys Lipids. 1995 Aug 1;77(1):65-78.
Membrane proteins are typically extracted by detergent concentrations of 0.5-2.0%, using detergent/protein ratios of 1:1 to 3:1. We have compared the ability of 14 different detergents from seven different structural and ionic classes, at a concentration of 2.0% and a detergent/protein ratio of 2:1, to extract an integral membrane protein (the serotonin 5-HT1A receptor) in active form and have observed profound differences in both lipids and proteins. All extracts were freed from detergents and dialyzed to form vesicles containing 95-100% of the extracted lipids, prior to [3H]8-hydroxy-2-(N,N-di-n-propylamino)tetralin ([3H]8-OH-DPAT) binding. The most efficient detergents in extracting active 5-HT1A receptor protein were the zwitterionic 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) and 3-[(cholamidopropyl)dimethylammonio]-2-hydroxy-1-propanesulfonate (CHAPSO), followed by the neutral n-dodecyl-beta-D-maltoside. Zwitterionic detergents also produced the highest solubilized lipid/protein ratio (3.0 and 2.5, respectively) and in general the relative amounts of extracted lipids and proteins followed inverse profiles. Thus, hydrophobic detergents such as Tritons (with critical micelle concentrations similar to CHAPS) and Thesit (structurally similar to Lubrol) extracted the most protein, but relatively little lipid (ratios of less than 0.2) and very little active 5-HT receptor. Dramatic differences were also observed in the ratios of individual lipids extracted by the same concentrations of different detergents and resolved by high-performance thin-layer chromatography. For example, galactosylceramide (GalCer) content ranged from 2.7% (CHAPSO) to 13.4% (sodium cholate) of the total lipid extract and cholesterol ranged from 0% (digitonin) to 17.9% (Triton X-100). The detergent-extractability profile for phosphatidylethanolamine (PE) (range 15-40% of total lipid) paralleled that of phosphatidylinositol (PI) (range 4-10%), but was inverse to that for GalCer and cholesterol. Detergent-extractability profiles for phosphatidylcholine (PC) and phosphatidylserine (PS) also followed inverse profiles, with zwitterionic detergents giving high PS/PC and high PE/PC ratios (approximately 2:1), whereas the Tritons and digitonin gave ratios of 1:2. We believe that differential solubilization of lipids, as well as proteins, by detergents is important for the biological activity of the extracted proteins, and lipid extractability should be taken into account when purifying membrane proteins.


