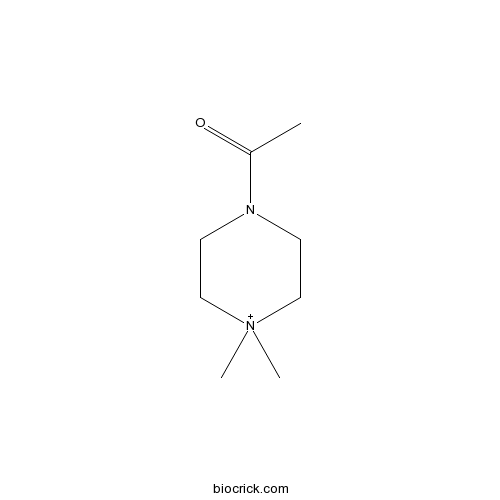
4-Acetyl-1,1-dimethylpiperazinium iodideNicotinic agonist CAS# 75667-84-4 |
- BCX 1470
Catalog No.:BCC1413
CAS No.:217099-43-9
- BCX 1470 methanesulfonate
Catalog No.:BCC1414
CAS No.:217099-44-0
- PMSF
Catalog No.:BCC1229
CAS No.:329-98-6
- Nafamostat Mesylate(FUT-175)
Catalog No.:BCC1228
CAS No.:82956-11-4
- Aprotinin
Catalog No.:BCC1220
CAS No.:9087-70-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 75667-84-4 | SDF | Download SDF |
| PubChem ID | 4392675 | Appearance | Powder |
| Formula | C8H17IN2O | M.Wt | 284.14 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | 1-(4,4-dimethylpiperazin-4-ium-1-yl)ethanone | ||
| SMILES | CC(=O)N1CC[N+](CC1)(C)C | ||
| Standard InChIKey | MSBLMBWXUVQCDY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H17N2O/c1-8(11)9-4-6-10(2,3)7-5-9/h4-7H2,1-3H3/q+1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Structural analog of acetylcholine that acts as a nicotinic agonist (Ki = 29.9 nM at α4β2). Upregulates the number of α4β2 binding sites in M10 cells in vitro by 440%. |

4-Acetyl-1,1-dimethylpiperazinium iodide Dilution Calculator

4-Acetyl-1,1-dimethylpiperazinium iodide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5194 mL | 17.597 mL | 35.1939 mL | 70.3878 mL | 87.9848 mL |
| 5 mM | 0.7039 mL | 3.5194 mL | 7.0388 mL | 14.0776 mL | 17.597 mL |
| 10 mM | 0.3519 mL | 1.7597 mL | 3.5194 mL | 7.0388 mL | 8.7985 mL |
| 50 mM | 0.0704 mL | 0.3519 mL | 0.7039 mL | 1.4078 mL | 1.7597 mL |
| 100 mM | 0.0352 mL | 0.176 mL | 0.3519 mL | 0.7039 mL | 0.8798 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Strobamine
Catalog No.:BCN1943
CAS No.:75656-91-6
- Boc-D-Asn-OH
Catalog No.:BCC3362
CAS No.:75647-01-7
- DSLET
Catalog No.:BCC5718
CAS No.:75644-90-5
- Chalcostrobamine
Catalog No.:BCN1900
CAS No.:75638-72-1
- Knightolamine
Catalog No.:BCN1912
CAS No.:75638-70-9
- Oncrasin 1
Catalog No.:BCC2390
CAS No.:75629-57-1
- Gomisin K1
Catalog No.:BCN7030
CAS No.:75629-20-8
- Moracenin B
Catalog No.:BCC8341
CAS No.:75629-19-5
- CHAPS
Catalog No.:BCC1476
CAS No.:75621-03-3
- (-)-Usnic acid
Catalog No.:BCN4306
CAS No.:7562-61-0
- (S)-(+)-α-Methylhistamine dihydrobromide
Catalog No.:BCC6700
CAS No.:75614-93-6
- Fludarabine Phosphate (Fludara)
Catalog No.:BCC3681
CAS No.:75607-67-9
- 2,4-Dihydroxy-4,6-dimethoxydihydrochalcone
Catalog No.:BCN1363
CAS No.:75679-58-2
- Isradipine (Dynacirc)
Catalog No.:BCC3797
CAS No.:75695-93-1
- 5-Amino-2-methylindole
Catalog No.:BCC8731
CAS No.:7570-49-2
- Leflunomide
Catalog No.:BCC1256
CAS No.:75706-12-6
- Eupalinilide B
Catalog No.:BCN2521
CAS No.:757202-08-7
- Eupalinilide C
Catalog No.:BCN2522
CAS No.:757202-11-2
- Eupalinilide D
Catalog No.:BCN2523
CAS No.:757202-14-5
- 17-AAG (KOS953)
Catalog No.:BCC2121
CAS No.:75747-14-7
- Cedrusin
Catalog No.:BCN4307
CAS No.:75775-36-9
- 3-Acetoxyflavone
Catalog No.:BCC9200
CAS No.:7578-68-9
- ADX 10059 hydrochloride
Catalog No.:BCC6171
CAS No.:757949-98-7
- Prosapogenin CP4
Catalog No.:BCN2534
CAS No.:75799-18-7
Regulation of nicotinic receptor subtypes following chronic nicotinic agonist exposure in M10 and SH-SY5Y neuroblastoma cells.[Pubmed:9572289]
J Neurochem. 1998 May;70(5):2028-37.
The present study further investigated whether nicotinic acetylcholine receptor (nAChR) subtypes differ in their ability to up-regulate following chronic exposure to nicotinic agonists. Seven nicotinic agonists were studied for their ability to influence the number of chick alpha4beta2 nAChR binding sites stably transfected in fibroblasts (M10 cells) following 3 days of exposure. The result showed a positive correlation between the Ki values for binding inhibition and EC50 values for agonist-induced alpha4beta2 nAChR up-regulation. The effects of epibatidine and nicotine were further investigated in human neuroblastoma SH-SY5Y cells (expressing alpha3, alpha5, beta2, and beta4 nAChR subunits). Nicotine exhibited a 14 times lower affinity for the nAChRs in SH-SY5Y cells as compared with M10 cells, whereas epibatidine showed similar affinities for the nAChRs expressed in the two cell lines. The nicotine-induced up-regulation of nAChR binding sites in SH-SY5Y cells was shifted to the right by two orders of magnitude as compared with that in M10 cells. The epibatidine-induced up-regulation of nAChR binding sites in SH-SY5Y cells was one-fourth that in M10 cells. The levels of mRNA of the various nAChR subunits were measured following the nicotinic agonist exposure. In summary, the various nAChR subtypes show different properties in their response to chronic stimulation.
Binding of semirigid nicotinic agonists to nicotinic and muscarinic receptors.[Pubmed:2747625]
Mol Pharmacol. 1989 Jul;36(1):177-84.
Isoarecolone methiodide (1-methyl-4-acetyl-1,2,3,6-tetrahydropyridine methiodide) was previously shown to be among the most potent agonists tested at the frog neuromuscular junction. Because nicotinic receptors from different sources vary in their selectivities, isoarecolone methiodide as well as 19 additional congeners, most of which were also previously tested at the frog neuromuscular junction, were studied in binding assays. Torpedo nobiliana was the tissue source for nicotinic receptors. Two types of experiments were conducted. The first evaluated the affinities of the agonists (including acetylcholine and carbamylcholine) for the recognition site by allowing the agonists to compete for that site with 125I-alpha-bungarotoxin. The inhibition potencies obtained correlated strongly (Spearman's correlation coefficient,-0.91) with the potency obtained at the frog neuromuscular junction. The second type of experiment evaluated the agonists for their ability to activate the receptor. The binding of [3H]perhydrohistrionicotoxin, which was employed as an indicator of the activation of the receptor, was measured in the presence of each of the agonists. Isoarecolone methiodide was the most potent of all. A few of the agonists (partial agonists) were incapable of fully enhancing this binding. For the full agonists, the concentration that produced half of the maximum binding of [3H]perhydrohistrionicotoxin was defined as the EC50. The correlation coefficient (Spearman's) for EC50 versus potency at the frog neuromuscular junction was -0.73, indicating innate differences between Torpedo and frog receptors. In addition, these compounds were tested for their affinity at muscarinic receptors from rat brain. Competition experiments were carried out using [3H]N-methylscopolamine. The affinity of isoarecolone methiodide was only about 7-fold lower than that of acetylcholine and less than 2-fold lower than that of carbamylcholine. In contrast, 1-methyl-4-acetylpiperazine methiodide was much more selective for nicotinic receptors. Its activity was similar to isoarecolone methiodide at the nicotinic receptor, but it was among the weakest compounds in its affinity for the muscarinic receptor.
Structural and electronic requirements for potent agonists at a nicotinic receptor.[Pubmed:3485051]
Eur J Pharmacol. 1986 Jan 14;120(1):127-31.
A new agonist, isoarecolone methiodide (1,1-dimethyl-4-acetyl-1,2,3,6-tetrahydropyridinium iodide) was tested at the frog neuromuscular junction. It was 50 times more potent than carbamylcholine, making it one of the most potent nicotinic agonists known. In addition, its cyclic structure and conjugated carbonyl bond endow it with near rigidity. An analogous compound, 1,1-dimethyl-4-acetylpiperazinium iodide, was synthesized because of its similar geometry and rigidity. It was 2.6 times as potent as carbamylcholine but only 0.053 times as potent as isoarecolone methiodide. Computer assisted molecular modeling and molecular orbital calculations revealed steric and electrostatic field differences between these two compounds.


