LeflunomideAHR agonist,immunosuppressive agent CAS# 75706-12-6 |
- StemRegenin 1 (SR1)
Catalog No.:BCC3637
CAS No.:1227633-49-9
- SU5416
Catalog No.:BCC1974
CAS No.:204005-46-9
- CH 223191
Catalog No.:BCC3896
CAS No.:301326-22-7
- ITE
Catalog No.:BCC3902
CAS No.:448906-42-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 75706-12-6 | SDF | Download SDF |
| PubChem ID | 3899 | Appearance | Powder |
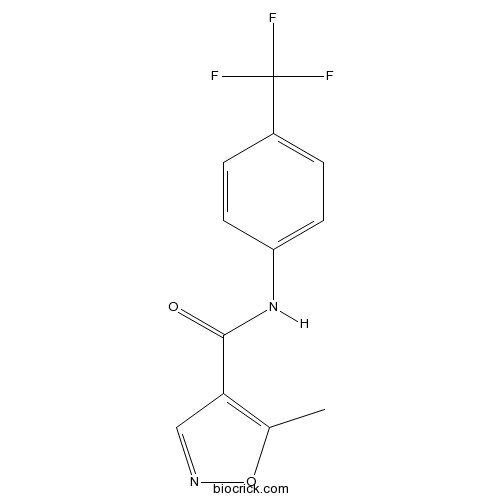
| Formula | C12H9F3N2O2 | M.Wt | 270.21 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | HWA 486, RS-34821 | ||
| Solubility | DMSO : ≥ 50 mg/mL (185.04 mM) Methanol : 2 mg/mL (7.40 mM; Need ultrasonic and warming) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 5-methyl-N-[4-(trifluoromethyl)phenyl]-1,2-oxazole-4-carboxamide | ||
| SMILES | CC1=C(C=NO1)C(=O)NC2=CC=C(C=C2)C(F)(F)F | ||
| Standard InChIKey | VHOGYURTWQBHIL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H9F3N2O2/c1-7-10(6-16-19-7)11(18)17-9-4-2-8(3-5-9)12(13,14)15/h2-6H,1H3,(H,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of dihydroorotate dehydrogenase (IC50 = 2.5 μM). Inhibits de novo pyrimidine synthesis in human T cells in vitro; also inhibits lymphocyte proliferation. Exhibits efficacy in several animal models of autoimmune disease, arthritis and graft rejection. Active metabolite, teriflunomide(A77 1726), also available. |

Leflunomide Dilution Calculator

Leflunomide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7008 mL | 18.5041 mL | 37.0083 mL | 74.0165 mL | 92.5206 mL |
| 5 mM | 0.7402 mL | 3.7008 mL | 7.4017 mL | 14.8033 mL | 18.5041 mL |
| 10 mM | 0.3701 mL | 1.8504 mL | 3.7008 mL | 7.4017 mL | 9.2521 mL |
| 50 mM | 0.074 mL | 0.3701 mL | 0.7402 mL | 1.4803 mL | 1.8504 mL |
| 100 mM | 0.037 mL | 0.185 mL | 0.3701 mL | 0.7402 mL | 0.9252 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Leflunomide is an agonist of aryl hydrocarbon receptor (AHR) [1]. As an immunoregulatory agent, Leflunomide can be administrated orally. A771726 (teriflunomide) is an active drug formed non-enzymatically from leflunomide [2].
Leflunomide has been demonstrated to expand Tregs levels in peripheral blood and stem cells subsets as well as to increase the migration of these cells into the injured kidney. Leflunomide has been displayed to increase cells expressed IL-10 and reduce cells expressed IL-17- and IL-23 in ischemic-reperfused kidney [1].
Leflunomide has shown to significantly lower the lipid peroxidation in male Wistar albino rats of sepsis. Meanwhile, leflunomide successfully inhibited the protein carbonyl rise and NO rise in bowel [2]. Leflunomide has shown to reduce cell proliferation, inhibit tumor marker expression, as well as down-regulate neuroendocrine marker ASCL1 protein expression in a dose- and time-dependent manner in human MTC-TT cells [3].
References:
[1] Baban B1, Liu JY, Mozaffari MS. Aryl hydrocarbon receptor agonist, leflunomide, protects the ischemic-reperfused kidney: role of Tregs and stem cells. Am J Physiol Regul Integr Comp Physiol. 2012 Dec;303(11):R1136-46.
[2] Ozturk E1, Surucu M2, Karaman A3, Samdancı E4, Fadillioglu E5. Protective effect of leflunomide against oxidative intestinal injury in a rodent model of sepsis. J Surg Res. 2014 Apr;187(2):610-5.
[3] Alhefdhi A1, Burke JF, Redlich A, Kunnimalaiyaan M, Chen H. Leflunomide suppresses growth in human medullary thyroid cancer cells. J Surg Res. 2013 Nov;185(1):212-6.
- 5-Amino-2-methylindole
Catalog No.:BCC8731
CAS No.:7570-49-2
- Isradipine (Dynacirc)
Catalog No.:BCC3797
CAS No.:75695-93-1
- 2,4-Dihydroxy-4,6-dimethoxydihydrochalcone
Catalog No.:BCN1363
CAS No.:75679-58-2
- 4-Acetyl-1,1-dimethylpiperazinium iodide
Catalog No.:BCC6616
CAS No.:75667-84-4
- Strobamine
Catalog No.:BCN1943
CAS No.:75656-91-6
- Boc-D-Asn-OH
Catalog No.:BCC3362
CAS No.:75647-01-7
- DSLET
Catalog No.:BCC5718
CAS No.:75644-90-5
- Chalcostrobamine
Catalog No.:BCN1900
CAS No.:75638-72-1
- Knightolamine
Catalog No.:BCN1912
CAS No.:75638-70-9
- Oncrasin 1
Catalog No.:BCC2390
CAS No.:75629-57-1
- Gomisin K1
Catalog No.:BCN7030
CAS No.:75629-20-8
- Moracenin B
Catalog No.:BCC8341
CAS No.:75629-19-5
- Eupalinilide B
Catalog No.:BCN2521
CAS No.:757202-08-7
- Eupalinilide C
Catalog No.:BCN2522
CAS No.:757202-11-2
- Eupalinilide D
Catalog No.:BCN2523
CAS No.:757202-14-5
- 17-AAG (KOS953)
Catalog No.:BCC2121
CAS No.:75747-14-7
- Cedrusin
Catalog No.:BCN4307
CAS No.:75775-36-9
- 3-Acetoxyflavone
Catalog No.:BCC9200
CAS No.:7578-68-9
- ADX 10059 hydrochloride
Catalog No.:BCC6171
CAS No.:757949-98-7
- Prosapogenin CP4
Catalog No.:BCN2534
CAS No.:75799-18-7
- Momordicoside A
Catalog No.:BCC8340
CAS No.:75801-95-5
- Dehydroevodiamine Chloride
Catalog No.:BCN6651
CAS No.:75853-60-0
- Rimcazole dihydrochloride
Catalog No.:BCC7090
CAS No.:75859-03-9
- 1-(3,5-Di-tert-butyl-4-hydroxyphenyl)-2-(2-(3-hydroxypropylamino)-5,6-dimethyl-1H-benzo[d]imidazol-1-yl)ethanone
Catalog No.:BCC1481
CAS No.:758679-97-9
Leflunomide prevents ROS-induced systemic fibrosis in mice.[Pubmed:28365359]
Free Radic Biol Med. 2017 Jul;108:192-203.
Systemic sclerosis (SSc) is a connective tissue disorder characterized by fibrosis of the skin and inner organs, vasculopathy and immunological abnormalities. Recent insights into the polarization of macrophages in scleroderma and into the implication of STAT6 and KLF4 in this process have prompted us to investigate the effects of the inhibition of STAT6 signaling pathway by Leflunomide in mice. SSc was induced in BALB/c mice by daily subcutaneous injections of hypochlorous acid (HOCl) or bleomycin. Mice were treated (or not) every other day, for 4 or 6 weeks, by Leflunomide. Skin and lung fibrosis as well as immunological features were studied. Mice exposed to HOCl developed a diffuse cutaneous SSc with pulmonary fibrosis and anti-DNA topoisomerase 1 auto-antibodies. STAT6 pathway was hyperactivated and KLF4 was overexpressed in the skin and the lungs of diseased mice. Their inhibition by Leflunomide prevented skin and lung fibrosis. Moreover, the hyperproliferative and pro-oxidative phenotype of skin and lung fibroblasts was reversed by Leflunomide. Beneficial immunological effects of Leflunomide were associated with decreased activation of CD4+ and CD8+ T cells, B cell activation, decreased auto-antibodies production and restored polarization of macrophages in the spleen. The improvement provided by Leflunomide in both mouse models of SSc provides a rationale for the evaluation of this immunomodulating drug in the management of patients affected by this disease.
Combination therapy of leflunomide and glucocorticoids for the maintenance of remission in patients with IgG4-related disease: a retrospective study and literature review.[Pubmed:28321964]
Intern Med J. 2017 Jun;47(6):680-689.
BACKGROUND: Although glucocorticoids are effective in IgG4-related disease (IgG4-RD), patients may relapse during or after glucocorticoid tapering. Immunosuppressive agents, including Leflunomide (LEF), are regarded as steroid-sparing agents in other autoimmune disorders and need to be discussed in the management of IgG4-RD. AIM: To identify the efficacy and safety of combination therapy of LEF and glucocorticoids in IgG4-RD. METHODS: We retrospectively summarised data of patients diagnosed with IgG4-RD between November 2012 and November 2015. Only patients treated with LEF plus glucocorticoids and had been followed up for more than three visits and 6 months were analysed with clinical symptoms, laboratory and imaging findings, treatment protocol, LEF-related adverse events and disease activity reflected by IgG4-RD Responder Index (IgG4-RD RI). RESULTS: A total of 18 patients, including 14 untreated patients and 4 relapsing patients, was included. The mean (SD) onset age was 54.0 (9.6) years. The mean (SD) follow-up period was 12.1 (7.4) months. All patients had active disease with mean (SD) IgG4-RD RI of 15.0 (5.6) at baseline and experienced improvements at 1 month. At the last follow up, the mean (SD) IgG4-RD Responder Index declined to 3.1 (1.7) in all patients and to 2.5 (1.2) in patients without relapse. The mean (SD) dose of GC declined to 6.9 (2.7) mg/day. A total of 12 (66.7%) and 11 (61.1%) patients were in remission at 6 months and the last follow up respectively. Three (16.7%) patients relapsed in clinical course. Two reversible adverse events were observed. CONCLUSION: The combination therapy of LEF and glucocoticoids is effective and safe in IgG4-RD.
Leflunomide for the treatment of trichodysplasia spinulosa in a liver transplant recipient.[Pubmed:28326649]
Transpl Infect Dis. 2017 Aug;19(4).
Trichodysplasia spinulosa (TS) is a rare dermatologic complication associated with the immunosuppressive therapy used in solid organ transplantation. The distinctive clinical manifestation of this condition is spiny follicular papules on the face, ears, extremities, and trunk. Histopathologically, abnormally maturing hair follicles with hyperkeratotic material are noted. The condition is produced by the trichodysplasia spinulosa-associated polyomavirus. Treatment of this condition in the past has entailed a reduction in immunosuppression, topical agents such as cidofovir or retinoids, or oral valganciclovir. Herein, we report a case of generalized TS treated successfully with Leflunomide.
Real-life experience of using conventional disease-modifying anti-rheumatic drugs (DMARDs) in psoriatic arthritis (PsA). Retrospective analysis of the efficacy of methotrexate, sulfasalazine, and leflunomide in PsA in comparison to spondyloarthritides other than PsA and literature review of the use of conventional DMARDs in PsA.[Pubmed:28293446]
Eur J Rheumatol. 2017 Mar;4(1):1-10.
OBJECTIVE: With the aim of assessing the response to treatment with conventional disease-modifying anti-rheumatic drugs (DMARDs) used in patients with psoriatic arthritis (PsA), data on methotrexate, sulfasalazine (SSZ), and Leflunomide were analyzed from baseline and subsequent follow-up (FU) questionnaires completed by patients with either PsA or other spondyloarthritides (SpAs). MATERIAL AND METHODS: A single-center real-life retrospective analysis was performed by obtaining clinical data via questionnaires administered before and after treatment. The indices used were erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) level, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Function Index (BASFI), wellbeing (WB), and treatment effect (TxE). The indices measured at baseline were compared with those measured on one occasion in a FU visit at least 1 year later. RESULTS: A total of 73 patients, 51 with PsA (mean age 49.8+/-12.8 years; male-to-female ratio [M:F]=18:33) and 22 with other SpAs (mean age 50.6+/-16 years; M:F=2:20), were studied. BASDAI, BASFI, and WB displayed consistent improvements during FU assessments in both PsA patients and controls in comparison to baseline values. SSZ exhibited better efficacy as confirmed by TxE in both PsA patients and controls. ESR and CRP displayed no differences in either the PsA or the SpA group between the cases before and after treatment. CONCLUSION: Real-life retrospective analysis of three DMARDs used in PsA (and SpAs other than PsA) demonstrated that all three DMARDs that were used brought about improvements in BASDAI, BASFI, TxE, and WB. However, the greatest improvements at FU were seen with SSZ use in both PsA and control cohorts.
Molecular mechanisms of action of new xenobiotic immunosuppressive drugs: tacrolimus (FK506), sirolimus (rapamycin), mycophenolate mofetil and leflunomide.[Pubmed:8902398]
Curr Opin Immunol. 1996 Oct;8(5):710-20.
Among all the new immunosuppressive molecules being investigated either preclinically or clinically, four stand out: tacrolimus (FK506), sirolimus (rapamycin), mycophenolate mofetil and Leflunomide (and its malononitriloamide analogs). Each drug has distinct mechanisms of immunosuppressive action, and in the past year significant advances have been made in our understanding of the actions of these drugs at the molecular and even atomic levels. Data from recent clinical trials demonstrate that these drugs very effectively suppress graft rejection or autoimmune diseases, validating the pivotal role played by each of their distinct molecular targets in the normal functioning of immune cells.
The immunosuppressant leflunomide inhibits lymphocyte proliferation by inhibiting pyrimidine biosynthesis.[Pubmed:7473131]
J Pharmacol Exp Ther. 1995 Nov;275(2):1043-9.
Leflunomide is a novel immunosuppressive compound that is effective in the treatment of animal models of autoimmune disease and human rheumatoid arthritis. The mechanism of action is unknown. Here we show that Leflunomide blocked 1) increases in nucleolar size and number, 2) upregulation of the nuclear protein antigens (PCNA and Ki-67), 3) increases in uridine incorporation and total RNA and DNA content, 4) cell cycle progression and 5) proliferation in mitogen-stimulated rat spleen mononuclear cells and human peripheral blood mononuclear cells (HPBMC). Exogenous uridine reversed the Leflunomide-dependent inhibition of the normal increase in total RNA and DNA content in mitogen-stimulated HPBMC and rat spleen cells. Uridine reversed the Leflunomide-dependent inhibition of cell cycle progression in stimulated rat cell cultures. Either uridine or cytidine, which can be converted to uridine by cytidine deaminase, reversed the antiproliferative effect of Leflunomide in HPBMC. Dihydroorotate accumulated in Leflunomide-treated human T-lymphoblastoid cells, suggesting that the compound inhibited the fourth enzyme in the pyrimidine biosynthetic pathway, dihydroorotate dehydrogenase. The results support the hypothesis that the in vitro effects of Leflunomide on T-lymphocytes are due to inhibition of de novo pyrimidine synthesis.
Inhibition of dihydroorotate dehydrogenase by the immunosuppressive agent leflunomide.[Pubmed:7575649]
Biochem Pharmacol. 1995 Sep 7;50(6):861-7.
Leflunomide [HWA 486 or RS-34821, 5-methyl-N-(4-trifluoromethylphenyl)-4-isoxazole carboximide] is an immunosuppressive agent effective in the treatment of rheumatoid arthritis. In spite of its clinical potential, its mechanism of action has not been elucidated. Recent studies suggest that Leflunomide may interfere with the metabolism of pyrimidine nucleotides. In our studies, the active metabolite of Leflunomide, RS-61980 (A77 1726, 2-hydroxyethylidene-cyanoacetic acid-4-trifluoromethyl anilide), was cytostatic towards a human T-lymphoblastoma cell line (A3.01). The inhibition of growth could be overcome completely by uridine. The other nucleosides, cytidine, adenosine and guanosine, did not overcome the effect of the compound. Since uridine is a precursor for the salvage synthesis of UMP, we propose that RS-61980 may be inhibiting the de novo pathway of UMP synthesis. Using human cells, the six enzymes catalyzing de novo UMP biosynthesis were tested for their sensitivity towards RS-61980. Only one of the enzymes, dihydroortate dehydrogenase (DHODH, EC 1.3.3.1) was inhibited by RS-61980 with a Ki value of 2.7 +/- 0.7 microM. The other five enzymes were not affected. The inhibition exhibited mixed-type kinetics towards both substrates, dihydroorotic acid and coenzyme Q. These results suggest that the molecular target of Leflunomide action is DHODH. The immunomodulating activity may be related to the inhibition of UMP synthesis in proliferating lymphocytes.


