Dehydroevodiamine ChlorideCAS# 75853-60-0 |
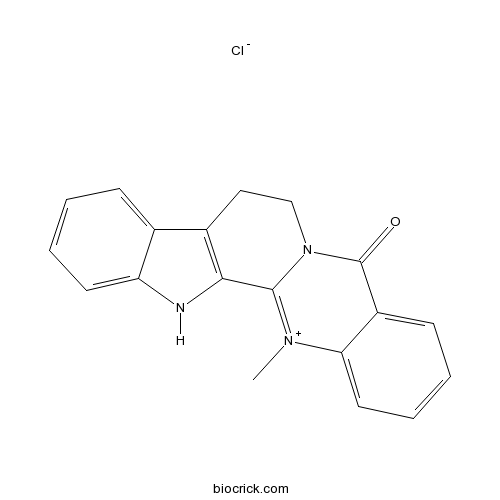
- Dehydroevodiamine hydrochloride
Catalog No.:BCN9178
CAS No.:111664-82-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 75853-60-0 | SDF | Download SDF |
| PubChem ID | 156371 | Appearance | Powder |
| Formula | C19H16ClN3O | M.Wt | 337.8 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CN1C2=CC=CC=C2C(=O)[N+]3=C1C4=C(CC3)C5=CC=CC=C5N4.[Cl-] | ||
| Standard InChIKey | SVOMSEHNGXLQRU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H15N3O.ClH/c1-21-16-9-5-3-7-14(16)19(23)22-11-10-13-12-6-2-4-8-15(12)20-17(13)18(21)22;/h2-9H,10-11H2,1H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dehydroevodiamine hydrochloride has novel anti-cholinesterase and antiamnesic activities, it inhibits acetylcholinesterase activity in a dose-dependent and non-competitive manner(IC50=37.8 microM); its potent antiamnesic effect is thought to be due to the combined effects of acetylcholinesterase inhibition and the known cerebral blood flow enhancement. Dehydroevodiamine hydrochloride (0.1-0.3 mg/kg iv) can increase the cerebral blood flow recorded from the surface of the supra-sylvian gyrus in anesthetized cats, suggest that it selectively increases cerebral blood flow. |
| Targets | AChR |
| In vivo | Increased feline cerebral blood flow induced by dehydroevodiamine hydrochloride from Evodia rutaecarpa.[Pubmed: 8201313]J Nat Prod. 1994 Mar;57(3):387-9.
|
| Animal Research | Novel anticholinesterase and antiamnesic activities of dehydroevodiamine, a constituent of Evodia rutaecarpa.[Pubmed: 8923803 ]Planta Med. 1996 Oct;62(5):405-9.
To find a new compound with antiamnesic activity, we screened 29 natural products for their abilities to inhibit acetylcholinesterase and reverse scopolamine-induced amnesia.
|

Dehydroevodiamine Chloride Dilution Calculator

Dehydroevodiamine Chloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9603 mL | 14.8017 mL | 29.6033 mL | 59.2066 mL | 74.0083 mL |
| 5 mM | 0.5921 mL | 2.9603 mL | 5.9207 mL | 11.8413 mL | 14.8017 mL |
| 10 mM | 0.296 mL | 1.4802 mL | 2.9603 mL | 5.9207 mL | 7.4008 mL |
| 50 mM | 0.0592 mL | 0.296 mL | 0.5921 mL | 1.1841 mL | 1.4802 mL |
| 100 mM | 0.0296 mL | 0.148 mL | 0.296 mL | 0.5921 mL | 0.7401 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Momordicoside A
Catalog No.:BCC8340
CAS No.:75801-95-5
- Prosapogenin CP4
Catalog No.:BCN2534
CAS No.:75799-18-7
- ADX 10059 hydrochloride
Catalog No.:BCC6171
CAS No.:757949-98-7
- 3-Acetoxyflavone
Catalog No.:BCC9200
CAS No.:7578-68-9
- Cedrusin
Catalog No.:BCN4307
CAS No.:75775-36-9
- 17-AAG (KOS953)
Catalog No.:BCC2121
CAS No.:75747-14-7
- Eupalinilide D
Catalog No.:BCN2523
CAS No.:757202-14-5
- Eupalinilide C
Catalog No.:BCN2522
CAS No.:757202-11-2
- Eupalinilide B
Catalog No.:BCN2521
CAS No.:757202-08-7
- Leflunomide
Catalog No.:BCC1256
CAS No.:75706-12-6
- 5-Amino-2-methylindole
Catalog No.:BCC8731
CAS No.:7570-49-2
- Isradipine (Dynacirc)
Catalog No.:BCC3797
CAS No.:75695-93-1
- Rimcazole dihydrochloride
Catalog No.:BCC7090
CAS No.:75859-03-9
- 1-(3,5-Di-tert-butyl-4-hydroxyphenyl)-2-(2-(3-hydroxypropylamino)-5,6-dimethyl-1H-benzo[d]imidazol-1-yl)ethanone
Catalog No.:BCC1481
CAS No.:758679-97-9
- Bay 65-1942 R form
Catalog No.:BCC1410
CAS No.:758683-21-5
- [Nle4,D-Phe7]-α-MSH
Catalog No.:BCC5963
CAS No.:75921-69-6
- 11-Hydroxycanthin-6-one
Catalog No.:BCN3104
CAS No.:75969-83-4
- Pancreatic Polypeptide (human)
Catalog No.:BCC5711
CAS No.:75976-10-2
- Camphor
Catalog No.:BCN8297
CAS No.:76-22-2
- Triamcinolone Acetonide
Catalog No.:BCC3871
CAS No.:76-25-5
- Oxymorphone
Catalog No.:BCC5255
CAS No.:76-41-5
- Bornyl isobutyrate
Catalog No.:BCC8134
CAS No.:50277-27-5
- Rhynchophylline
Catalog No.:BCN4979
CAS No.:76-66-4
- Neoquassine
Catalog No.:BCN3120
CAS No.:76-77-7
Increased feline cerebral blood flow induced by dehydroevodiamine hydrochloride from Evodia rutaecarpa.[Pubmed:8201313]
J Nat Prod. 1994 Mar;57(3):387-9.
Dehydroevodiamine hydrochloride (0.1-0.3 mg/kg iv), which was isolated from the leaves of Evodia rutaecarpa, increased the cerebral blood flow recorded from the surface of the supra-sylvian gyrus in anesthetized cats. This action reached a maximum 1-4 min after injection and continued for 10 min. However, the compound had negligible effects on other cardiorespiratory functions at the doses examined. These results suggest that the compound selectively increases cerebral blood flow.
Novel anticholinesterase and antiamnesic activities of dehydroevodiamine, a constituent of Evodia rutaecarpa.[Pubmed:8923803]
Planta Med. 1996 Oct;62(5):405-9.
To find a new compound with antiamnesic activity, we screened 29 natural products for their abilities to inhibit acetylcholinesterase and reverse scopolamine-induced amnesia. Among the plants tested Evodia rutaecarpa Bentham showed a strong inhibitory effect on acetylcholinesterase in vitro and an anti-amnesic effect in vivo. By sequential fractionation of E. rutaecarpa, the active component was finally identified as dehydroevodiamine hydrochloride (DHED). DHED inhibited acetylcholinesterase activity in a dose-dependent and non-competitive manner. The IC50 value of DHED is 37.8 microM. A single administration of DHED to rats (6.25 mg/kg) significantly reversed the scopolamine-induced memory impairment in a passive avoidance test. The antiamnesic effect of DHED was more potent than that of tacrine which is the only drug for Alzheimer's disease approved by FDA. This potent antiamnesic effect of DHED was thought to be due to the combined effects of acetylcholinesterase inhibition and the known cerebral blood flow enhancement. These results indicate that DHED has novel anti-cholinesterase and antiamnesic activities and might have therapeutic potential in various disorders including Alzheimer's disease.


