17-AAG (KOS953)Hsp90 inhibitor CAS# 75747-14-7 |
- KW-2478
Catalog No.:BCC2127
CAS No.:819812-04-9
- NVP-BEP800
Catalog No.:BCC2129
CAS No.:847559-80-2
- IPI-504 (Retaspimycin hydrochloride)
Catalog No.:BCC2126
CAS No.:857402-63-2
- PU-H71
Catalog No.:BCC1872
CAS No.:873436-91-0
- SNX-2112
Catalog No.:BCC2132
CAS No.:908112-43-6
- MPC-3100
Catalog No.:BCC2128
CAS No.:958025-66-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 75747-14-7 | SDF | Download SDF |
| PubChem ID | 22524980 | Appearance | Powder |
| Formula | C31H43N3O8 | M.Wt | 585.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NSC 330507, 17-(Allylamino)-17-demethoxygeldanamycin, Tanespimycin | ||
| Solubility | DMSO : ≥ 55 mg/mL (93.91 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
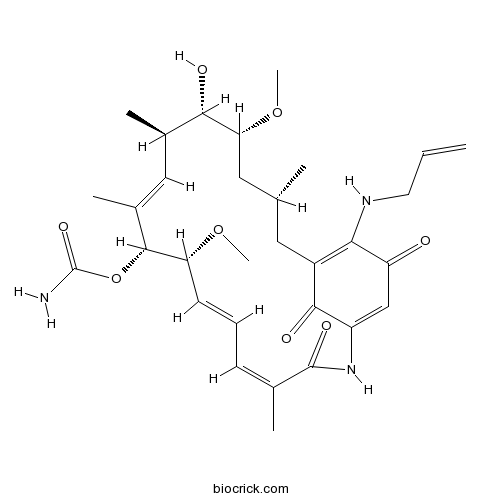
| Chemical Name | [(3R,5R,6S,7R,8E,10R,11R,12E,14Z)-6-hydroxy-5,11-dimethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-21-(prop-2-enylamino)-17-azabicyclo[16.3.1]docosa-1(21),8,12,14,18-pentaen-10-yl] carbamate | ||
| SMILES | CC1CC(C(C(C=C(C(C(C=CC=C(C(=O)NC2=CC(=O)C(=C(C1)C2=O)NCC=C)C)OC)OC(=O)N)C)C)O)OC | ||
| Standard InChIKey | AYUNIORJHRXIBJ-CQFHKJEWSA-N | ||
| Standard InChI | InChI=1S/C31H43N3O8/c1-8-12-33-26-21-13-17(2)14-25(41-7)27(36)19(4)15-20(5)29(42-31(32)39)24(40-6)11-9-10-18(3)30(38)34-22(28(21)37)16-23(26)35/h8-11,15-17,19,24-25,27,29,33,36H,1,12-14H2,2-7H3,(H2,32,39)(H,34,38)/b11-9+,18-10-,20-15+/t17-,19-,24-,25-,27+,29-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of heat shock protein 90 (Hsp90) chaperone activity, and an analog of geldanamycin. Subsequently inhibits the activity of oncogenic proteins such as p185erbB-2 (IC50 = 31 nM), N-ras, Ki-ras and c-Akt. Antitumor in vivo. Also protects neuroprogenitor cells against stress-induced apoptosis at low concentrations (10 nM) in vitro. |

17-AAG (KOS953) Dilution Calculator

17-AAG (KOS953) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7074 mL | 8.5368 mL | 17.0736 mL | 34.1472 mL | 42.684 mL |
| 5 mM | 0.3415 mL | 1.7074 mL | 3.4147 mL | 6.8294 mL | 8.5368 mL |
| 10 mM | 0.1707 mL | 0.8537 mL | 1.7074 mL | 3.4147 mL | 4.2684 mL |
| 50 mM | 0.0341 mL | 0.1707 mL | 0.3415 mL | 0.6829 mL | 0.8537 mL |
| 100 mM | 0.0171 mL | 0.0854 mL | 0.1707 mL | 0.3415 mL | 0.4268 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
17-AAG induced BAX-dependent apoptosis at pharmacologically relevant concentrations in BAX knockout HCT116 human colon carcinoma cells both in vitro and in tumor xenografts in vivo, where 17-AAG predominantly inhibited cell proliferation rather than promoting cell death.
Abstract
17-AAG is a naturally occurring inhibitor of HSP90 that binds to HSP90 and hence induces the degradation of its client proteins.
Abstract
17-AAG, an inhibitor of HSP90, was screened for its anti-melanoma activity.
Abstract
A serious disadvantage in the promising Paclitaxel/17-AAG combination cancer therapy is the requirement of large quantities of toxic organic surfactants and solvents to solubilize the drug.
Abstract
17-AAG induced the down-regulation of AXL expression in a time- and dose-dependent manner through promoting AXL polyubiquitinlation and subsequent proteasomal degradation, in which 17-AAG requires AXL intracellular domain regardless of AXL receptor phosphorylation.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
17-AAG is a potent inhibitor of HSP90 with IC50 value of 6 nM in BT474 cells [1].
17-AAG is a synthetic analogue developed from geldanamycin which was found to have significant hepatic toxicity. 17-AAG has an improved toxicity profile and has no hepatic toxicity. 17-AAG can bind to HSP90 and destabilize the client proteins such as HER2, Raf-1, p53 and MAPK signaling. In Multiple myeloma (MM) cells, 17-AAG treatment inhibited cell proliferation and survival. The combination treatment of 17-AAG and bortezomib induced apoptosis in primary MM cells resistant to doxorubicin and bortezomib. The combination of 17-AAG and trastuzumab reduced the expression of ErbB2 in breast cancer cells overexpressing ErbB2. 17-AAG also showed efficacy in thyroid cancer cells and Hodgkin lymphoma cells. Besides that, 17-AAG was found to increased apoptosis in human melanoma xenografts. 17-AAG is now in phase II clinical studies [2].
References:
[1] Kamal A, Thao L, Sensintaffar J, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors[J]. Nature, 2003, 425(6956): 407-410..
[2] Dimopoulos M A, Mitsiades C S, Anderson K C, et al. Tanespimycin as antitumor therapy. Clinical Lymphoma Myeloma and Leukemia, 2011, 11(1): 17-22
- Eupalinilide D
Catalog No.:BCN2523
CAS No.:757202-14-5
- Eupalinilide C
Catalog No.:BCN2522
CAS No.:757202-11-2
- Eupalinilide B
Catalog No.:BCN2521
CAS No.:757202-08-7
- Leflunomide
Catalog No.:BCC1256
CAS No.:75706-12-6
- 5-Amino-2-methylindole
Catalog No.:BCC8731
CAS No.:7570-49-2
- Isradipine (Dynacirc)
Catalog No.:BCC3797
CAS No.:75695-93-1
- 2,4-Dihydroxy-4,6-dimethoxydihydrochalcone
Catalog No.:BCN1363
CAS No.:75679-58-2
- 4-Acetyl-1,1-dimethylpiperazinium iodide
Catalog No.:BCC6616
CAS No.:75667-84-4
- Strobamine
Catalog No.:BCN1943
CAS No.:75656-91-6
- Boc-D-Asn-OH
Catalog No.:BCC3362
CAS No.:75647-01-7
- DSLET
Catalog No.:BCC5718
CAS No.:75644-90-5
- Chalcostrobamine
Catalog No.:BCN1900
CAS No.:75638-72-1
- Cedrusin
Catalog No.:BCN4307
CAS No.:75775-36-9
- 3-Acetoxyflavone
Catalog No.:BCC9200
CAS No.:7578-68-9
- ADX 10059 hydrochloride
Catalog No.:BCC6171
CAS No.:757949-98-7
- Prosapogenin CP4
Catalog No.:BCN2534
CAS No.:75799-18-7
- Momordicoside A
Catalog No.:BCC8340
CAS No.:75801-95-5
- Dehydroevodiamine Chloride
Catalog No.:BCN6651
CAS No.:75853-60-0
- Rimcazole dihydrochloride
Catalog No.:BCC7090
CAS No.:75859-03-9
- 1-(3,5-Di-tert-butyl-4-hydroxyphenyl)-2-(2-(3-hydroxypropylamino)-5,6-dimethyl-1H-benzo[d]imidazol-1-yl)ethanone
Catalog No.:BCC1481
CAS No.:758679-97-9
- Bay 65-1942 R form
Catalog No.:BCC1410
CAS No.:758683-21-5
- [Nle4,D-Phe7]-α-MSH
Catalog No.:BCC5963
CAS No.:75921-69-6
- 11-Hydroxycanthin-6-one
Catalog No.:BCN3104
CAS No.:75969-83-4
- Pancreatic Polypeptide (human)
Catalog No.:BCC5711
CAS No.:75976-10-2
Protection of murine neural progenitor cells by the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin in the low nanomolar concentration range.[Pubmed:21395580]
J Neurochem. 2011 May;117(4):703-11.
Stem cell-based approaches provide hope as a potential therapy for neurodegenerative diseases and stroke. One of the major scientific hurdles for stem cell therapy is the poor survival rate of the newly formed or transplanted neural stem cells. In this study, we found that low-dose treatment with the Heat shock protein 90 (Hsp90) inhibitor 17-allylamino-17-demethoxygeldanamycin (17-AAG), a heavily investigated anti-cancer drug, prevented neural progenitor cells from either naturally-occurring or stress-induced apoptosis, although it induced apoptosis at higher doses. This stress adaptation effect mediated by low-dose 17-AAG is accompanied by activation of multiple cell survival pathways, including the stress response pathway (induction of Hsp70), the MAPK pathway, and the PI3K/Akt pathway. When administered in vivo, 17-AAG led to Akt and glycogen synthase kinase 3beta phosphorylation, and more 5-bromo-2'-deoxyuridine positive cells in the mouse brain. These findings could have profound implications in stem cell therapy for neurodegenerative diseases and stroke.
Inhibition of signal transduction by the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin results in cytostasis and apoptosis.[Pubmed:11358818]
Cancer Res. 2001 May 15;61(10):4003-9.
17-Allylamino-17-demethoxygeldanamycin (17AAG) is a first-in-class heat shock protein 90 (Hsp90) molecular chaperone inhibitor to enter clinical trials. The downstream molecular and cellular consequences of Hsp90 inhibition are not well defined. 17AAG has shown activity against human colon cancer in cell culture and xenograft models. In this study, we demonstrated that in addition to depleting c-Raf-1 and inhibiting ERK-1/2 phosphorylation in human colon adenocarcinoma cells, 17AAG also depleted N-ras, Ki-ras, and c-Akt and inhibited phosphorylation of c-AKT: A consequence of these events was the induction of cell line-dependent cytostasis and apoptosis, although the latter did not result from dephosphorylation of proapoptotic BAD: One cell line, KM12, did not exhibit apoptosis and in contrast to the other cell lines overexpressed Bag-1, but did not express BAX: Taken together with other determinants of 17AAG sensitivity, these results should contribute to a more complete understanding of the molecular pharmacology of 17AAG, which in turn should aid the future rational clinical development and use of the drug in colon and other tumor types.
Disruption of the EF-2 kinase/Hsp90 protein complex: a possible mechanism to inhibit glioblastoma by geldanamycin.[Pubmed:11358819]
Cancer Res. 2001 May 15;61(10):4010-6.
Glioblastoma multiforme is the most treatment-resistant brain tumor. Elongation factor-2 (EF-2) kinase (calmodulin kinase III) is a unique protein kinase that is overexpressed in glioma cell lines and in human surgical specimens. Several mitogens activate this kinase and inhibitors block mitogen activation and produce cell death. Geldanamycin (GA) is a benzoquinone ansamycin antibiotic that disrupts Hsp90-protein interactions. Because EF-2 kinase is chaperoned by Hsp90, we investigated the effects of GA on the viability of glioma cells, the expression of EF-2 kinase protein, and the interaction between Hsp90 and EF-2 kinase. GA was a potent inhibitor of the clonogenicity of four glioma cells lines with IC(50)s ranging from 1 to 3 nM. 17-allylamino-17-demethoxygeldanamycin (17-AAG), a less toxic and less potent derivative of GA, inhibited the clonogenicity of glioma cells with IC(50) values of 13 nM in C6 cells and 35 nM in T98G cells. Treatment of cell lines for 24-48 h of GA or 17-AAG disrupted EF-2-kinase/Hsp90 interactions as measured by coimmunoprecipitation, resulting in a decreased amount of recoverable kinase in cell lysates. The ability of GA to inhibit the growth of glioma cells was abrogated by overexpressing EF-2 kinase. In addition, 17-AAG significantly inhibited the growth of a glioma xenograft in nude mice. These studies demonstrate for the first time the activity of GAs against human gliomas in vitro and in vivo and suggest that destruction of EF-2 kinase may be an important cytotoxic mechanism of this unique class of drug.
Inhibition of the oncogene product p185erbB-2 in vitro and in vivo by geldanamycin and dihydrogeldanamycin derivatives.[Pubmed:7562911]
J Med Chem. 1995 Sep 15;38(19):3806-12.
The erbB-2 oncogene encodes a transmembrane protein tyrosine kinase which plays a pivotal role in signal transduction and has been implicated when overexpressed in breast, ovarian, and gastric cancers. Naturally occurring benzoquinoid ansamycin antibiotics herbimycin A, geldanamycin (GDM), and dihydrogeldanamycin were found to potently deplete p185, the erbB-2 oncoprotein, in human breast cancer SKBR-3 cells in culture. Chemistry efforts to modify selectively the quinoid moiety of GDM afforded derivatives with greater potency in vitro and in vivo. Analogs demonstrated inhibition of p185 phosphotyrosine in cell culture and in vivo after systemic drug administration to nu/nu nude mice bearing Fisher rat embryo cells transfected with human erbB-2 (FRE/erbB-2). Specifically, dosed intraperitoneally at 100 mg/kg, 17-(allylamino)-17-demethoxygeldanamycin and other 17-amino analogs were effective at reducing p185 phosphotyrosine in subcutaneous flank FRE/erbB-2 tumors. Modifications to the 17-19-positions of the quinone ring revealed a broad structure-activity relationship in vitro.


